当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Auto Tandem Catalysis: Asymmetric Vinylogous Cycloaddition/Kinetic Resolution Sequence for the Enantioselective Synthesis of Spiro-Dihydropyranone from Benzylidene Meldrum's Acid
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-27 , DOI: 10.1002/adsc.202100559 martial TOFFANO 1 , Régis Guillot 2 , Chloee BOURNAUD 2 , jean-François Brière 3 , Giang Vo-Thanh 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-27 , DOI: 10.1002/adsc.202100559 martial TOFFANO 1 , Régis Guillot 2 , Chloee BOURNAUD 2 , jean-François Brière 3 , Giang Vo-Thanh 2
Affiliation
|
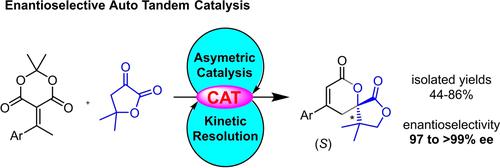
A catalytic enantioselective vinylogous domino reaction has been achieved from ketone-derived benzylidene Meldrum's acid and α-ketolactones to provide spirolactone dihydropyranones with more than 99% ee. An Auto Tandem Catalysis (ATC) process resulting from dual and complementary role of (DHQ)2PHAL organocatalyst resulted in a sequence involving an asymmetric vinylogous formal (4+2) cycloaddition of benzylidene and the subsequent kinetic resolution operating through a 1,3-prototropic shift leading to good yields (>50%) and the high selectivity.
中文翻译:

自动串联催化:从亚苄基 Meldrum 酸对映选择性合成螺二氢吡喃酮的不对称乙烯基环加成/动力学拆分序列
已经从酮衍生的亚苄基 Meldrum 酸和 α-酮内酯实现了催化对映选择性乙烯基多米诺反应,以提供具有超过 99% ee 的螺内酯二氢吡喃酮。由 (DHQ) 2 PHAL 有机催化剂的双重和互补作用产生的自动串联催化 (ATC) 过程产生了一个序列,涉及亚苄基的不对称乙烯基形式 (4+2) 环加成和随后通过 1,3- 操作的动力学拆分质子转移导致良好的产率(> 50%)和高选择性。
更新日期:2021-09-21
中文翻译:

自动串联催化:从亚苄基 Meldrum 酸对映选择性合成螺二氢吡喃酮的不对称乙烯基环加成/动力学拆分序列
已经从酮衍生的亚苄基 Meldrum 酸和 α-酮内酯实现了催化对映选择性乙烯基多米诺反应,以提供具有超过 99% ee 的螺内酯二氢吡喃酮。由 (DHQ) 2 PHAL 有机催化剂的双重和互补作用产生的自动串联催化 (ATC) 过程产生了一个序列,涉及亚苄基的不对称乙烯基形式 (4+2) 环加成和随后通过 1,3- 操作的动力学拆分质子转移导致良好的产率(> 50%)和高选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号