当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Easy, Convenient, and Safe Process for the Synthesis of Lofexidine Hydrochloride
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.oprd.0c00453 Monica Donnola 1 , Annalisa Airoldi 1 , Alessandro Barozza 1 , Jacopo Roletto 1 , Paolo Paissoni 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.oprd.0c00453 Monica Donnola 1 , Annalisa Airoldi 1 , Alessandro Barozza 1 , Jacopo Roletto 1 , Paolo Paissoni 1
Affiliation
|
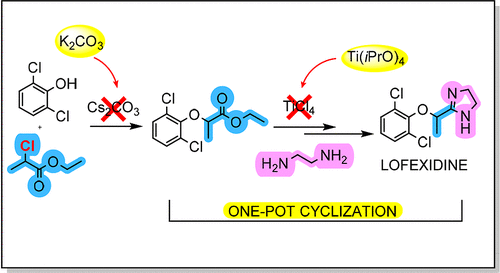
A very efficient, cost-effective, and easily scalable process for the synthesis of lofexidine hydrochloride (1), an alpha 2-adrenergic receptor agonist used for treating opioid withdrawal is presented. Process development allows the preparation of lofexidine hydrochloride (1) through a one-pot amidation/imidazoline ring formation reaction, starting from ethyl 2-(2,6-dichlorophenoxy)propionate (13) and ethylenediamine (5) by the action of titanium isopropoxide. The required intermediate ethyl 2-(2,6-dichlorophenoxy)propionate (13) can efficiently be obtained through O-alkylation of 2,6-dichlorophenol (2) with ethyl 2-chloropropionate (12) using potassium carbonate as an acid-scavenger agent.
中文翻译:

一种简单、方便、安全的盐酸洛非西定合成工艺
提出了一种非常有效、经济且易于扩展的合成盐酸洛非西丁 ( 1 ) 的方法,该方法是一种用于治疗阿片类药物戒断的 α2-肾上腺素能受体激动剂。工艺开发允许从 2-(2,6-二氯苯氧基)丙酸乙酯 ( 13 ) 和乙二胺 ( 5 ) 在异丙醇钛的作用下,通过一锅酰胺化/咪唑啉成环反应制备盐酸洛非西丁 ( 1 ) . 所需的中间体2-(2,6-二氯苯氧基)丙酸乙酯( 13 )可以通过2-氯丙酸乙酯( 12 )对2,6-二氯苯酚( 2 )的O-烷基化反应得到。) 使用碳酸钾作为酸清除剂。
更新日期:2021-08-20
中文翻译:

一种简单、方便、安全的盐酸洛非西定合成工艺
提出了一种非常有效、经济且易于扩展的合成盐酸洛非西丁 ( 1 ) 的方法,该方法是一种用于治疗阿片类药物戒断的 α2-肾上腺素能受体激动剂。工艺开发允许从 2-(2,6-二氯苯氧基)丙酸乙酯 ( 13 ) 和乙二胺 ( 5 ) 在异丙醇钛的作用下,通过一锅酰胺化/咪唑啉成环反应制备盐酸洛非西丁 ( 1 ) . 所需的中间体2-(2,6-二氯苯氧基)丙酸乙酯( 13 )可以通过2-氯丙酸乙酯( 12 )对2,6-二氯苯酚( 2 )的O-烷基化反应得到。) 使用碳酸钾作为酸清除剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号