当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of the Plasmodium falciparum heat-shock protein 70-x ATPase domain in complex with chemical fragments identify conserved and unique binding sites
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-07-28 , DOI: 10.1107/s2053230x21007378 Nada Mohamad 1 , Ailsa O'Donoghue 1 , Anastassia L Kantsadi 1 , Ioannis Vakonakis 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-07-28 , DOI: 10.1107/s2053230x21007378 Nada Mohamad 1 , Ailsa O'Donoghue 1 , Anastassia L Kantsadi 1 , Ioannis Vakonakis 1
Affiliation
|
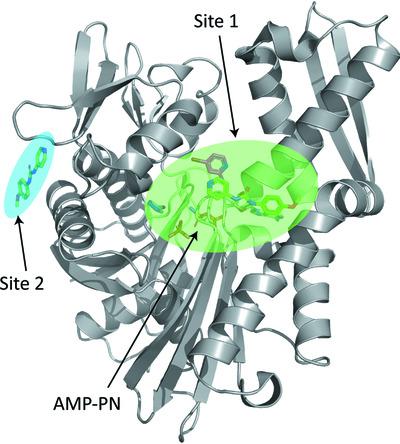
Plasmodium falciparum invades erythrocytes and extensively modifies them in a manner that increases the virulence of this malaria parasite. A single heat-shock 70 kDa-type chaperone, PfHsp70-x, is among the parasite proteins exported to the host cell. PfHsp70-x assists in the formation of a key protein complex that underpins parasite virulence and supports parasite growth during febrile episodes. Previous work resolved the crystallographic structures of the PfHsp70-x ATPase and substrate-binding domains, and showed them to be highly similar to those of their human counterparts. Here, 233 chemical fragments were screened for binding to the PfHsp70-x ATPase domain, resulting in three crystallographic structures of this domain in complex with ligands. Two binding sites were identified, with most ligands binding proximal to the ATPase nucleotide-binding pocket. Although amino acids participating in direct ligand interactions are conserved between the parasite and human erythrocytic chaperones, one nonconserved residue is also present near the ligand. This work suggests that PfHsp70-x features binding sites that may be exploitable by small-molecule ligands towards the specific inhibition of the parasite chaperone.
中文翻译:

恶性疟原虫热休克蛋白 70-x ATPase 结构域与化学片段复合物的结构鉴定出保守且独特的结合位点
恶性疟原虫侵入红细胞并以增加这种疟疾寄生虫毒力的方式广泛改变红细胞。单一热休克 70 kDa 型伴侣 PfHsp70-x 是输出到宿主细胞的寄生虫蛋白之一。PfHsp70-x 有助于形成关键的蛋白质复合物,该复合物增强寄生虫的毒力并支持寄生虫在发热期间的生长。先前的工作解析了 PfHsp70-x ATP 酶和底物结合域的晶体结构,并表明它们与人类对应物高度相似。在此,筛选了 233 个化学片段以与 PfHsp70-x ATPase 结构域结合,从而产生该结构域与配体复合物的三种晶体结构。鉴定出两个结合位点,大多数配体结合在 ATP 酶核苷酸结合袋附近。尽管参与直接配体相互作用的氨基酸在寄生虫和人红细胞伴侣之间是保守的,但配体附近也存在一个非保守残基。这项工作表明,PfHsp70-x 具有可被小分子配体利用的结合位点,以特异性抑制寄生虫伴侣。
更新日期:2021-08-04
中文翻译:

恶性疟原虫热休克蛋白 70-x ATPase 结构域与化学片段复合物的结构鉴定出保守且独特的结合位点
恶性疟原虫侵入红细胞并以增加这种疟疾寄生虫毒力的方式广泛改变红细胞。单一热休克 70 kDa 型伴侣 PfHsp70-x 是输出到宿主细胞的寄生虫蛋白之一。PfHsp70-x 有助于形成关键的蛋白质复合物,该复合物增强寄生虫的毒力并支持寄生虫在发热期间的生长。先前的工作解析了 PfHsp70-x ATP 酶和底物结合域的晶体结构,并表明它们与人类对应物高度相似。在此,筛选了 233 个化学片段以与 PfHsp70-x ATPase 结构域结合,从而产生该结构域与配体复合物的三种晶体结构。鉴定出两个结合位点,大多数配体结合在 ATP 酶核苷酸结合袋附近。尽管参与直接配体相互作用的氨基酸在寄生虫和人红细胞伴侣之间是保守的,但配体附近也存在一个非保守残基。这项工作表明,PfHsp70-x 具有可被小分子配体利用的结合位点,以特异性抑制寄生虫伴侣。









































 京公网安备 11010802027423号
京公网安备 11010802027423号