当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The identification and structural analysis of potential 14-3-3 interaction sites on the bone regulator protein Schnurri-3
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-07-28 , DOI: 10.1107/s2053230x21006658 Lorenzo Soini 1 , Seppe Leysen 2 , Tom Crabbe 3 , Jeremy Davis 4 , Christian Ottmann 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-07-28 , DOI: 10.1107/s2053230x21006658 Lorenzo Soini 1 , Seppe Leysen 2 , Tom Crabbe 3 , Jeremy Davis 4 , Christian Ottmann 1
Affiliation
|
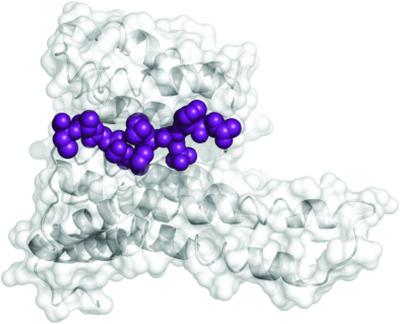
14-3-3 proteins regulate many intracellular processes and their ability to bind in subtly different fashions to their numerous partner proteins provides attractive drug-targeting points for a range of diseases. Schnurri-3 is a suppressor of mouse bone formation and a candidate target for novel osteoporosis therapeutics, and thus it is of interest to determine whether it interacts with 14-3-3. In this work, potential 14-3-3 interaction sites on mammalian Schnurri-3 were identified by an in silico analysis of its protein sequence. Using fluorescence polarization, isothermal titration calorimetry and X-ray crystallography, it is shown that synthetic peptides containing either phosphorylated Thr869 or Ser542 can indeed interact with 14-3-3, with the latter capable of forming an interprotein disulfide bond with 14-3-3σ: a hitherto unreported phenomenon.
中文翻译:

骨调节蛋白 Schnurri-3 上潜在 14-3-3 相互作用位点的鉴定和结构分析
14-3-3 蛋白调节许多细胞内过程,它们以细微不同的方式与其众多伙伴蛋白结合的能力为一系列疾病提供了有吸引力的药物靶向点。Schnurri-3 是小鼠骨形成的抑制剂,也是新型骨质疏松症治疗的候选靶点,因此确定它是否与 14-3-3 相互作用是很有意义的。在这项工作中,通过对其蛋白质序列进行计算机分析,鉴定了哺乳动物 Schnurri-3 上潜在的 14-3-3 相互作用位点 。使用荧光偏振、等温滴定量热法和 X 射线晶体学,表明含有磷酸化 Thr869 或 Ser542 的合成肽确实可以与 14-3-3 相互作用,后者能够与 14-3- 形成蛋白间二硫键3σ:迄今为止尚未报道的现象。
更新日期:2021-08-04
中文翻译:

骨调节蛋白 Schnurri-3 上潜在 14-3-3 相互作用位点的鉴定和结构分析
14-3-3 蛋白调节许多细胞内过程,它们以细微不同的方式与其众多伙伴蛋白结合的能力为一系列疾病提供了有吸引力的药物靶向点。Schnurri-3 是小鼠骨形成的抑制剂,也是新型骨质疏松症治疗的候选靶点,因此确定它是否与 14-3-3 相互作用是很有意义的。在这项工作中,通过对其蛋白质序列进行计算机分析,鉴定了哺乳动物 Schnurri-3 上潜在的 14-3-3 相互作用位点 。使用荧光偏振、等温滴定量热法和 X 射线晶体学,表明含有磷酸化 Thr869 或 Ser542 的合成肽确实可以与 14-3-3 相互作用,后者能够与 14-3- 形成蛋白间二硫键3σ:迄今为止尚未报道的现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号