当前位置:
X-MOL 学术
›
ACS ES&T Water
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of Iron(IV) in the Oxidation of Amines by Ferrate(VI): Theoretical Insight and Implications in Oxidizing Pharmaceuticals
ACS ES&T Water ( IF 4.8 ) Pub Date : 2021-07-27 , DOI: 10.1021/acsestwater.1c00156 J. Clayton Baum 1 , Mingbao Feng 2 , Binglin Guo 2 , Ching-Hua Huang 3 , Virender K. Sharma 2
ACS ES&T Water ( IF 4.8 ) Pub Date : 2021-07-27 , DOI: 10.1021/acsestwater.1c00156 J. Clayton Baum 1 , Mingbao Feng 2 , Binglin Guo 2 , Ching-Hua Huang 3 , Virender K. Sharma 2
Affiliation
|
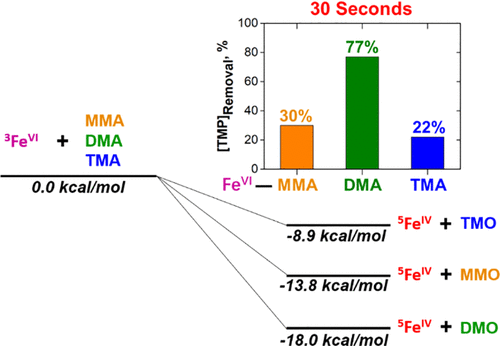
Aliphatic amines are ubiquitously present in natural waters and constitute one of the major moieties in dissolved organic matter and water micropollutants such as pharmaceuticals. This paper presents aliphatic amine {monomethylamine (CH3NH2, MMA), dimethylamine [(CH3)2NH, DMA], and trimethylamine [(CH3)3N, TMA]}-enhanced oxidation of pharmaceuticals (trimethoprim, atenolol, carbamazepine, and sulfadiazine) by ferrate(VI) [FeVIO42–, Fe(VI)]. The magnitude of the enhancement varies with amines, and DMA shows the greatest potential to increase the level of oxidation of trimethoprim. The computational approach is applied to describe the trend of an increased level of oxidation by Fe(VI) in the presence of amines. The computation showed that an Fe(VI)/amine solution forms highly reactive FeIV species as intermediates that react with pharmaceuticals to yield enhanced oxidation. In the rate-determining step of Fe(VI) reactions with amines, TS1, the Fe(VI) is reduced and abstracts an H from N, followed by the transfer of an O atom to N to form a very stable intermediate involving a nitroxide radical. The abstracted H is then transferred back to the N-O group in TS2 to form an H-bonded product complex, which dissociates to the products. The difference in the reactivity of the FeIV species with the type of amine explains the trend seen experimentally in the enhanced oxidation of pharmaceuticals.
中文翻译:

铁(IV)在胺的高铁酸盐的氧化生成(VI):理论洞察和在氧化制药启示
脂族胺是在自然水域遍存在并构成溶解的有机物质和水微量的主要部分中的一个,如药品。本文呈现的脂族胺{甲胺(CH 3 NH 2,MMA),二甲基胺[(CH 3)2 NH,DMA],和三甲胺[(CH 3)3 N,TMA]} -增强药物的氧化(甲氧苄啶,阿替洛尔,卡马西平,和磺胺嘧啶)通过高铁酸盐(VI)的[Fe VI Ò 4 2-,铁(VI)]。增强的幅度与胺变化,以及DMA显示增加甲氧苄啶氧化水平的最大潜力。计算方法适用于描述的氧化在胺存在水平增加的Fe(VI)的趋势。计算表明,Fe(VI)/胺溶液形成高反应性的 Fe IV种作为与药物以得到增强的氧化反应的中间体。在Fe的速率决定步骤(VI)与胺反应,TS1,铁(VI)被还原并提取选自N为H,接着O原子为N的转移,以形成非常稳定的中间体,涉及氮氧激进的。然后该抽象H被转移回NO组中TS2,以形成H-粘结产物复合物,其解离产物。在Fe的反应性的差异IV物质与胺的类型说明了药物的增强的氧化实验观察到的趋势。
更新日期:2021-08-13
中文翻译:

铁(IV)在胺的高铁酸盐的氧化生成(VI):理论洞察和在氧化制药启示
脂族胺是在自然水域遍存在并构成溶解的有机物质和水微量的主要部分中的一个,如药品。本文呈现的脂族胺{甲胺(CH 3 NH 2,MMA),二甲基胺[(CH 3)2 NH,DMA],和三甲胺[(CH 3)3 N,TMA]} -增强药物的氧化(甲氧苄啶,阿替洛尔,卡马西平,和磺胺嘧啶)通过高铁酸盐(VI)的[Fe VI Ò 4 2-,铁(VI)]。增强的幅度与胺变化,以及DMA显示增加甲氧苄啶氧化水平的最大潜力。计算方法适用于描述的氧化在胺存在水平增加的Fe(VI)的趋势。计算表明,Fe(VI)/胺溶液形成高反应性的 Fe IV种作为与药物以得到增强的氧化反应的中间体。在Fe的速率决定步骤(VI)与胺反应,TS1,铁(VI)被还原并提取选自N为H,接着O原子为N的转移,以形成非常稳定的中间体,涉及氮氧激进的。然后该抽象H被转移回NO组中TS2,以形成H-粘结产物复合物,其解离产物。在Fe的反应性的差异IV物质与胺的类型说明了药物的增强的氧化实验观察到的趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号