当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organosilanes in Metal-Catalyzed, Enantioselective Reductions
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.oprd.1c00073 Gerald L. Larson 1 , Richard J. Liberatore 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.oprd.1c00073 Gerald L. Larson 1 , Richard J. Liberatore 2
Affiliation
|
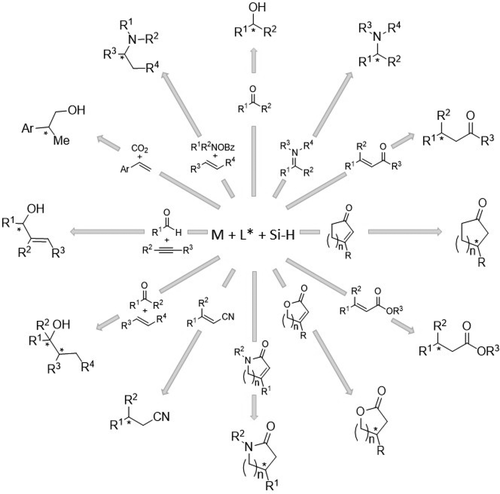
The growth and development of an extensive range of metals complexed with chiral ligands for the purpose of catalyzing a variety of reactions in an enantioselective manner has been impressive for its scope, chemical yields and high ee values. Chief among the classes of synthetic transformations has been that of the asymmetric reduction of prochiral substrates. These include the asymmetric reduction of prochiral ketones, imines, unsaturated aldehydes, ketones, esters, nitriles and olefins, as well as a number of asymmetric coupling reactions. In concert with these efficient catalyst systems, organosilanes have the ability to carry out any number of organic reductions under a variety of conditions. In these reactions, which in reality are hydrosilylations (hydrosilylation and reduction are considered interchangeable herein) in which the initially resulting silylated product is hydrolyzed to the ultimate desired functionality, the ability to sterically and electronically alter the organosilane reductant can contribute to the overall success of the transformation. In this review, we present a thorough compilation of the literature covering the use of organosilanes in metal-catalyzed asymmetric reductions (hydrosilylations) and coupling reactions.
中文翻译:

金属催化对映选择性还原中的有机硅烷
为了以对映选择性方式催化各种反应,大量与手性配体络合的金属的生长和发展因其范围、化学产率和高 ee 值而令人印象深刻。合成转化类别中最主要的是前手性底物的不对称还原。这些包括前手性酮、亚胺、不饱和醛、酮、酯、腈和烯烃的不对称还原,以及许多不对称偶联反应。与这些高效的催化剂体系相结合,有机硅烷能够在各种条件下进行任意数量的有机还原。在这些反应中,这实际上是氢化硅烷化(氢化硅烷化和还原在本文中被认为是可互换的),其中最初得到的硅烷化产物被水解为最终所需的官能度,空间和电子改变有机硅烷还原剂的能力有助于转化的整体成功。在这篇综述中,我们对涵盖有机硅烷在金属催化不对称还原(氢化硅烷化)和偶联反应中使用的文献进行了全面汇编。
更新日期:2021-08-20
中文翻译:

金属催化对映选择性还原中的有机硅烷
为了以对映选择性方式催化各种反应,大量与手性配体络合的金属的生长和发展因其范围、化学产率和高 ee 值而令人印象深刻。合成转化类别中最主要的是前手性底物的不对称还原。这些包括前手性酮、亚胺、不饱和醛、酮、酯、腈和烯烃的不对称还原,以及许多不对称偶联反应。与这些高效的催化剂体系相结合,有机硅烷能够在各种条件下进行任意数量的有机还原。在这些反应中,这实际上是氢化硅烷化(氢化硅烷化和还原在本文中被认为是可互换的),其中最初得到的硅烷化产物被水解为最终所需的官能度,空间和电子改变有机硅烷还原剂的能力有助于转化的整体成功。在这篇综述中,我们对涵盖有机硅烷在金属催化不对称还原(氢化硅烷化)和偶联反应中使用的文献进行了全面汇编。











































 京公网安备 11010802027423号
京公网安备 11010802027423号