Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.reactfunctpolym.2021.105000 Yantus A.B. Neolaka 1 , Yosep Lawa 1 , Johnson Naat 1 , Arsel A.P. Riwu 2 , Yeskiel E. Lindu 2 , Handoko Darmokoesoemo 3 , Bernadeta Ayu Widyaningrum 4 , Munawar Iqbal 5 , Heri Septya Kusuma 6
|
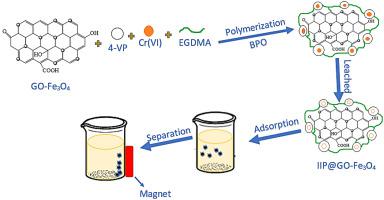
Magnetic composite material modified ion imprinting polymer (IIP@GO-Fe3O4) based on GO from kesambi wood (Schleichera oleosa) was successfully synthesized by using a precipitation method. The physico-chemical characterization uses XRD, FTIR, BET-BJH and SEM-EDX. IIP@GO-Fe3O4 was applied to adsorb Cr(VI) from the water sample by utilizing the batch system. Various adsorption parameters for Cr(VI) such as adsorbent mass, pH, contact time and temperature were optimized in this investigation. The Cr(VI) maximum adsorption occurred when the adsorbent mass usage 0.04 g, pH 2, 40 min of contacts and at the temperature of 323 K. In this study ten kinetic and eight isothermal adsorption models were used to investigate the adsorption mechanism of Cr(VI) onto IIP@GO-Fe3O4. The kinetic modelling shows that the adsorption of Cr(VI) onto IIP@GO-Fe3O4 corresponds to the pseudo-second-order (PSO) model, while the adsorption isotherm corresponds to the Dubinin-Radushkevich (DKR) model with an adsorption capacity of 8.502 mg/g. The thermodynamic study has shown that the adsorption process can take place at a temperature of 323 K. The result showed that a higher adsorption selectivity on IIP@GO-Fe3O4 compares to NIP@GO-Fe3O4. The adsorbent materials gave better reusability because their adsorptive capacity remained at the same level (although it was used at least ten times).
中文翻译:

基于 Kesambi wood (Schleichera oleosa) 的磁性材料 IIP@GO-Fe3O4 作为从水溶液中去除 Cr(VI) 的潜在吸附剂的评估
磁性复合材料改性离子印迹聚合物(IIP @ GO-的Fe 3 ö 4)的基础上从GO木材kesambi(Schleichera oleosa) 成功地通过使用沉淀法合成。物理化学表征使用 XRD、FTIR、BET-BJH 和 SEM-EDX。IIP@GO-Fe 3 O 4用于利用间歇系统从水样中吸附 Cr(VI)。本研究优化了 Cr(VI) 的各种吸附参数,如吸附剂质量、pH、接触时间和温度。当吸附剂用量为 0.04 g、pH 为 2、接触时间为 40 min 且温度为 323 K 时,Cr(VI) 的吸附量最大。本研究使用 10 个动力学模型和 8 个等温吸附模型来研究 Cr 的吸附机理(VI) 到 IIP@GO-Fe 3 O 4 上。动力学模型表明 Cr(VI) 吸附到 IIP@GO-Fe 3 O 4对应于伪二级 (PSO) 模型,而吸附等温线对应于 Dubinin-Radushkevich (DKR) 模型,吸附容量为 8.502 mg/g。热力学研究表明,吸附过程可以在显示323 K.结果的温度发生,关于IIP较高吸附选择性@ GO-的Fe 3 ö 4进行比较,以NIP @ GO-的Fe 3种ö 4.吸附剂材料由于它们的吸附能力保持在同一水平(尽管至少使用了十次),因此具有更好的可重用性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号