当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on Ciprofibrate Equilibrium Solubility and Thermodynamic Correlation in Four Aqueous Cosolvency Mixtures at Saturation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.jced.1c00276 Guangyu Xu 1 , Fanyuan Zhang 1 , Zhenghui Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.jced.1c00276 Guangyu Xu 1 , Fanyuan Zhang 1 , Zhenghui Li 1
Affiliation
|
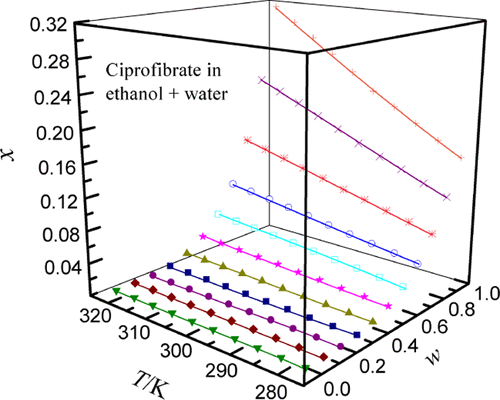
Drug solubility in the evaluated solvent systems plays an important role in the selection of lead substances in the pre-period of pharmaceutical development and discovery. This work involved five pure solvents as raw materials and formed four sets of mixed solvents according to their pairs by mixing ethylene glycol (EG), ethanol, n-propanol, and N,N-dimethyl formamide (DMF) in water. Taking 278.15 K as the starting temperature and referring to the principle of the isothermal static state, the temperature is gradually increased at 5 K intervals until it reaches 323.15 K. Under normal pressure (101.1 kPa), when the mole fraction of the solute did not change, the value obtained by HPLC was the equilibrium solubility of ciprofibrate. The obtained ciprofibrate solubility values were described mathematically by the Jouyban–Acree model, Van’t Hoff–Jouyban–Acree model, and modified Apelblat–Jouyban–Acree model, and the maximums of RAD and rmsd obtained from the three cosolvency models were 1.43 × 10–2 and 8.15 × 10–4, respectively. The solubility order circumstances of ciprofibrate at the temperature range of 278.15–323.15 K were as follows: water (2.653 × 10–6 at 323.15 K) < EG (8.227 × 10–2 at 323.15 K) < n-propanol (0.1693 at 323.15 K) < ethanol (0.3123 at 323.15 K) < DMF (0.4075 at 323.15 K). From the point of view of temperature, the influence on the solute cannot be ignored. The amount of ciprofibrate dissolved from a low value to a high value with the temperature from low to high. In addition, the difference was that the water content of the mixed solvent was repulsive to the dissolution of the solute. When the water content increased from low to high, the mole fraction of ciprofibrate decreased from high to low. The obtained model parameters gave the best description of the ciprofibrate solubility behaviors, and the Jouyban–Acree model could be fitted with good results. All of the achieved values of ciprofibrate in this study provide useful information for the purification and recrystallization processes in pharmaceutical sciences.
中文翻译:

环丙贝特在饱和状态下四种水性共溶剂混合物的平衡溶解度和热力学相关性研究
药物在被评估溶剂系统中的溶解度在药物开发和发现前期对先导物质的选择起着重要作用。本工作以五种纯溶剂为原料,将乙二醇(EG)、乙醇、正丙醇和N , N混合形成四套混合溶剂。-二甲基甲酰胺(DMF)在水中。以 278.15 K 为起始温度,参照等温静态原理,以 5 K 的间隔逐渐升温,直至达到 323.15 K。 在常压(101.1 kPa)下,当溶质的摩尔分数不变化,HPLC 得到的值为环丙贝特的平衡溶解度。获得的环丙贝特溶解度值通过 Jouyban-Acree 模型、Van't Hoff-Jouyban-Acree 模型和修正的 Apelblat-Jouyban-Acree 模型进行数学描述,从三个共溶解度模型中获得的 RAD 和 rmsd 的最大值为 1.43 × 10 –2和 8.15 × 10 –4, 分别。环丙贝特在 278.15–323.15 K 温度范围内的溶解度顺序情况如下:水 (2.653 × 10 –6 at 323.15 K) < EG (8.227 × 10 –2 at 323.15 K) < n-丙醇(323.15 K 时 0.1693)< 乙醇(323.15 K 时 0.3123)< DMF(323.15 K 时 0.4075)。从温度上看,对溶质的影响不可忽视。随着温度从低到高,环丙贝特的溶解量从低值到高值。此外,不同之处在于混合溶剂的含水量对溶质的溶解有排斥作用。当水含量由低到高时,环丙贝特的摩尔分数由高到低降低。获得的模型参数对环丙贝特的溶解行为给出了最好的描述,Jouyban-Acree 模型可以得到很好的结果。本研究中环丙贝特的所有实现值都为制药科学中的纯化和重结晶过程提供了有用的信息。
更新日期:2021-08-12
中文翻译:

环丙贝特在饱和状态下四种水性共溶剂混合物的平衡溶解度和热力学相关性研究
药物在被评估溶剂系统中的溶解度在药物开发和发现前期对先导物质的选择起着重要作用。本工作以五种纯溶剂为原料,将乙二醇(EG)、乙醇、正丙醇和N , N混合形成四套混合溶剂。-二甲基甲酰胺(DMF)在水中。以 278.15 K 为起始温度,参照等温静态原理,以 5 K 的间隔逐渐升温,直至达到 323.15 K。 在常压(101.1 kPa)下,当溶质的摩尔分数不变化,HPLC 得到的值为环丙贝特的平衡溶解度。获得的环丙贝特溶解度值通过 Jouyban-Acree 模型、Van't Hoff-Jouyban-Acree 模型和修正的 Apelblat-Jouyban-Acree 模型进行数学描述,从三个共溶解度模型中获得的 RAD 和 rmsd 的最大值为 1.43 × 10 –2和 8.15 × 10 –4, 分别。环丙贝特在 278.15–323.15 K 温度范围内的溶解度顺序情况如下:水 (2.653 × 10 –6 at 323.15 K) < EG (8.227 × 10 –2 at 323.15 K) < n-丙醇(323.15 K 时 0.1693)< 乙醇(323.15 K 时 0.3123)< DMF(323.15 K 时 0.4075)。从温度上看,对溶质的影响不可忽视。随着温度从低到高,环丙贝特的溶解量从低值到高值。此外,不同之处在于混合溶剂的含水量对溶质的溶解有排斥作用。当水含量由低到高时,环丙贝特的摩尔分数由高到低降低。获得的模型参数对环丙贝特的溶解行为给出了最好的描述,Jouyban-Acree 模型可以得到很好的结果。本研究中环丙贝特的所有实现值都为制药科学中的纯化和重结晶过程提供了有用的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号