当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Saturated Solubility Determination and Correlation of 2-Methyl-4-Methylamino-6-Methoxy-1,3,5-Triazine in Several Aqueous Cosolvent Systems from 278.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.jced.1c00288 Gaoquan Chen 1 , Sha Liu 1 , Jie Zhu 1 , Renjie Xu 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.jced.1c00288 Gaoquan Chen 1 , Sha Liu 1 , Jie Zhu 1 , Renjie Xu 2
Affiliation
|
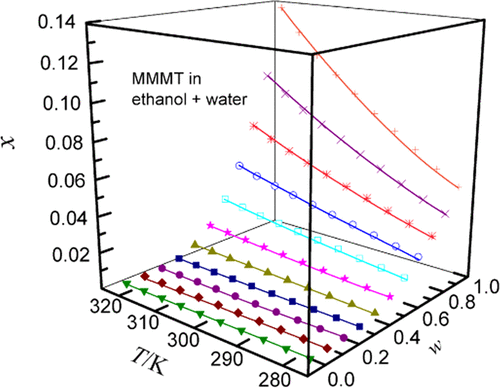
2-Methyl-4-methylamino-6-methoxy-1,3,5-triazine is an important intermediate of tribenuron-methyl with poor aqueous solubility. In this study, we have considered five pure solvents as raw materials and formed four sets of mixed solvents according to their pairs by mixing methanol, ethanol, ethylene glycol (EG), and N,N-dimethyl formamide (DMF) in water. Taking 278.15 K as the starting temperature and referring to the principle of the isothermal static state, the temperature was gradually increased at 5 K intervals until it reached 323.15 K. Under normal pressure (101.1 kPa), when the mole fraction of the solute did not change, the value obtained by high-performance liquid chromatography (HPLC) was the equilibrium solubility of 2-methyl-4-methylamino-6-methoxy-1,3,5-triazine. The mole fraction of 2-methyl-4-methylamino-6-methoxy-1,3,5-triazine at 323.15 K obeyed the following descending sequence: DMF (0.4557) > methanol (0.1852) > ethanol (0.1364) > EG (0.05972). From the point of view of temperature, the influence on the solute cannot be ignored. The amount of 2-methyl-4-methylamino-6-methoxy-1,3,5-triazine dissolved increased from a low value to a high value when the temperature increased from low to high. In addition, a difference observed was that the water content of the mixed solvent was repulsive to the dissolution of the solute. When the water content increased from low to high, the mole fraction of 2-methyl-4-methylamino-6-methoxy-1,3,5-triazine decreased from high to low. In any temperature environment, the same proportion of water was added to the four organic solvents. Compared with other solvents, the mixture of DMF and water had an absolute dissolution advantage. We adopted three cosolvency models including the Jouyban–Acree, van’t Hoff–Jouyban–Acree, and modified Apelblat–Jouyban–Acree models to calculate and correlate the saturated solubility data of 2-methyl-4-methylamino-6-methoxy-1,3,5-triazine. The RAD and RMSD values were 8.90 × 10–3 and 6.36 × 10–4, respectively, as obtained from the van’t Hoff–Jouyban–Acree model, and the Jouyban–Acree model demonstrated a better fitting effect. The determination of solubility helps to obtain solubility parameters, which are a measure of intermolecular forces. Solubility is required in the fields of recrystallization and fractional crystallization in the chemical industry, as well as the synthesis and separation of compounds.
中文翻译:

2-Methyl-4-Methylamino-6-Methoxy-1,3,5-Triazine 在 278.15 到 323.15 K 的几种水性共溶剂体系中的饱和溶解度测定和相关性
2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪是苯脲甲基的重要中间体,水溶性较差。本研究以五种纯溶剂为原料,将甲醇、乙醇、乙二醇(EG)、N,N按其对组合成四套混合溶剂。-二甲基甲酰胺(DMF)在水中。以 278.15 K 为起始温度,参照等温静态原理,以 5 K 的间隔逐渐升温,直至达到 323.15 K。 在常压(101.1 kPa)下,当溶质的摩尔分数不变化,高效液相色谱(HPLC)得到的值为2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪的平衡溶解度。2-甲基-4-甲氨基-6-甲氧基-1,3,5-三嗪在 323.15 K 的摩尔分数遵循以下递减顺序:DMF (0.4557) > 甲醇 (0.1852) > 乙醇 (0.1364) > EG (0.05972) )。从温度上看,对溶质的影响不可忽视。2-甲基-4-甲氨基-6-甲氧基-1,3的用量,当温度由低到高时,5-三嗪的溶解量由低值增加到高值。此外,观察到的差异是混合溶剂的水含量排斥溶质的溶解。当含水量由低到高时,2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪的摩尔分数由高到低降低。在任何温度环境下,四种有机溶剂中加入相同比例的水。与其他溶剂相比,DMF和水的混合物具有绝对的溶解优势。我们采用了三个共溶解度模型,包括 Jouyban-Acree、van't Hoff-Jouyban-Acree 和修正的 Apelblat-Jouyban-Acree 模型来计算和关联 2-methyl-4-methylamino-6-methoxy-1 的饱和溶解度数据,3,5-三嗪。–3和 6.36 × 10 –4分别是从 van't Hoff-Jouyban-Acree 模型获得的,Jouyban-Acree 模型表现出更好的拟合效果。溶解度的测定有助于获得溶解度参数,这是分子间作用力的量度。化学工业中的重结晶和分级结晶以及化合物的合成和分离等领域都需要溶解度。
更新日期:2021-08-12
中文翻译:

2-Methyl-4-Methylamino-6-Methoxy-1,3,5-Triazine 在 278.15 到 323.15 K 的几种水性共溶剂体系中的饱和溶解度测定和相关性
2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪是苯脲甲基的重要中间体,水溶性较差。本研究以五种纯溶剂为原料,将甲醇、乙醇、乙二醇(EG)、N,N按其对组合成四套混合溶剂。-二甲基甲酰胺(DMF)在水中。以 278.15 K 为起始温度,参照等温静态原理,以 5 K 的间隔逐渐升温,直至达到 323.15 K。 在常压(101.1 kPa)下,当溶质的摩尔分数不变化,高效液相色谱(HPLC)得到的值为2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪的平衡溶解度。2-甲基-4-甲氨基-6-甲氧基-1,3,5-三嗪在 323.15 K 的摩尔分数遵循以下递减顺序:DMF (0.4557) > 甲醇 (0.1852) > 乙醇 (0.1364) > EG (0.05972) )。从温度上看,对溶质的影响不可忽视。2-甲基-4-甲氨基-6-甲氧基-1,3的用量,当温度由低到高时,5-三嗪的溶解量由低值增加到高值。此外,观察到的差异是混合溶剂的水含量排斥溶质的溶解。当含水量由低到高时,2-甲基-4-甲基氨基-6-甲氧基-1,3,5-三嗪的摩尔分数由高到低降低。在任何温度环境下,四种有机溶剂中加入相同比例的水。与其他溶剂相比,DMF和水的混合物具有绝对的溶解优势。我们采用了三个共溶解度模型,包括 Jouyban-Acree、van't Hoff-Jouyban-Acree 和修正的 Apelblat-Jouyban-Acree 模型来计算和关联 2-methyl-4-methylamino-6-methoxy-1 的饱和溶解度数据,3,5-三嗪。–3和 6.36 × 10 –4分别是从 van't Hoff-Jouyban-Acree 模型获得的,Jouyban-Acree 模型表现出更好的拟合效果。溶解度的测定有助于获得溶解度参数,这是分子间作用力的量度。化学工业中的重结晶和分级结晶以及化合物的合成和分离等领域都需要溶解度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号