Immunity ( IF 25.5 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.immuni.2021.07.003 Nicole R Wong 1 , Jay Mohan 1 , Benjamin J Kopecky 1 , Shuchi Guo 1 , Lixia Du 2 , Jamison Leid 1 , Guoshuai Feng 1 , Inessa Lokshina 1 , Oleksandr Dmytrenko 1 , Hannah Luehmann 3 , Geetika Bajpai 1 , Laura Ewald 1 , Lauren Bell 1 , Nikhil Patel 4 , Andrea Bredemeyer 1 , Carla J Weinheimer 1 , Jessica M Nigro 1 , Attila Kovacs 1 , Sachio Morimoto 5 , Peter O Bayguinov 6 , Max R Fisher 6 , W Tom Stump 6 , Michael Greenberg 6 , James A J Fitzpatrick 7 , Slava Epelman 8 , Daniel Kreisel 9 , Rajan Sah 1 , Yongjian Liu 3 , Hongzhen Hu 2 , Kory J Lavine 10
|
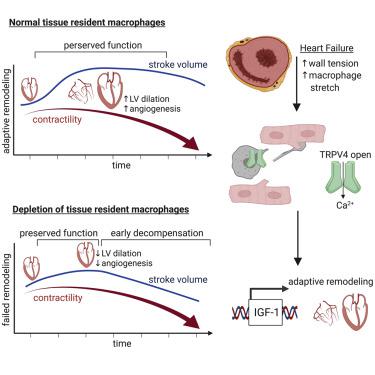
Cardiac macrophages represent a heterogeneous cell population with distinct origins, dynamics, and functions. Recent studies have revealed that C-C Chemokine Receptor 2 positive (CCR2+) macrophages derived from infiltrating monocytes regulate myocardial inflammation and heart failure pathogenesis. Comparatively little is known about the functions of tissue resident (CCR2−) macrophages. Herein, we identified an essential role for CCR2− macrophages in the chronically failing heart. Depletion of CCR2− macrophages in mice with dilated cardiomyopathy accelerated mortality and impaired ventricular remodeling and coronary angiogenesis, adaptive changes necessary to maintain cardiac output in the setting of reduced cardiac contractility. Mechanistically, CCR2− macrophages interacted with neighboring cardiomyocytes via focal adhesion complexes and were activated in response to mechanical stretch through a transient receptor potential vanilloid 4 (TRPV4)-dependent pathway that controlled growth factor expression. These findings establish a role for tissue-resident macrophages in adaptive cardiac remodeling and implicate mechanical sensing in cardiac macrophage activation.
中文翻译:

常驻心脏巨噬细胞介导适应性心肌重塑
心脏巨噬细胞代表具有不同起源、动力学和功能的异质细胞群。最近的研究表明,源自浸润单核细胞的 CC 趋化因子受体 2 阳性 (CCR2 + ) 巨噬细胞可调节心肌炎症和心力衰竭发病机制。对于组织驻留(CCR2 - )巨噬细胞的功能知之甚少。在此,我们确定了 CCR2 -巨噬细胞在慢性衰竭心脏中的重要作用。患有扩张型心肌病的小鼠中,CCR2-巨噬细胞的耗竭会加速死亡率,并损害心室重塑和冠状血管生成,这是在心肌收缩力降低的情况下维持心输出量所必需的适应性变化。从机制上讲, CCR2-巨噬细胞通过粘着斑复合物与邻近的心肌细胞相互作用,并通过控制生长因子表达的瞬时受体电位香草酸 4 (TRPV4) 依赖性途径响应机械拉伸而被激活。这些发现确立了组织驻留巨噬细胞在适应性心脏重塑中的作用,并暗示机械传感在心脏巨噬细胞激活中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号