当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The use of umbilical cord-derived mesenchymal stem cells in patients with muscular dystrophies: Results from compassionate use in real-life settings
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-07-27 , DOI: 10.1002/sctm.21-0027 Beata Świątkowska-Flis 1, 2 , Izabela Zdolińska-Malinowska 3 , Dominika Sługocka 1 , Dariusz Boruczkowski 3
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-07-27 , DOI: 10.1002/sctm.21-0027 Beata Świątkowska-Flis 1, 2 , Izabela Zdolińska-Malinowska 3 , Dominika Sługocka 1 , Dariusz Boruczkowski 3
Affiliation
|
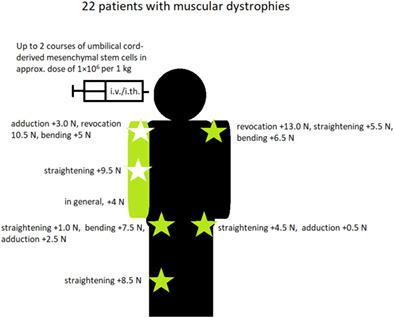
Muscular dystrophies are genetically determined progressive diseases with no cause-related treatment and limited supportive treatment. Although stem cells cannot resolve the underlying genetic conditions, their wide-ranging therapeutic properties may ameliorate the consequences of the involved mutations (oxidative stress, inflammation, mitochondrial dysfunction, necrosis). In this study, we administered advanced therapy medicinal product containing umbilical cord-derived mesenchymal stem cells (UC-MSCs) to 22 patients with muscular dystrophies. Patients received one to five intravenous and/or intrathecal injections per treatment course in up to two courses every 2 months. Four standard doses of 10, 20, 30, or 40 × 106 UC-MSCs per injection were used; the approximate dose per kilogram was 1 × 106 UC-MSCs. Muscle strength was measured with a set of CQ Dynamometer computerized force meters (CQ Elektronik System, Czernica, Poland). Statistical analysis of muscle strength in the whole group showed significant improvement in the right upper limb (+4.0 N); left hip straightening (+4.5 N) and adduction (+0.5 N); right hip straightening (+1.0 N), bending (+7.5 N), and adduction (+2.5 N); right knee straightening (+8.5 N); left shoulder revocation (+13.0 N), straightening (+5.5 N), and bending (+6.5 N); right shoulder adduction (+3.0 N), revocation (+10.5 N), and bending (+5 N); and right elbow straightening (+9.5 N); all these differences were statistically significant. In six patients (27.3%) these changes led to improvement in gait analysis or movement scale result. Only one patient experienced transient headache and lower back pain after the last administration. In conclusion, UC-MSC therapy may be considered as a therapeutic option for these patients.
中文翻译:

脐带来源间充质干细胞在肌营养不良患者中的应用:在现实生活中富有同情心使用的结果
肌营养不良症是遗传决定的进行性疾病,没有病因相关的治疗和有限的支持治疗。尽管干细胞不能解决潜在的遗传条件,但它们广泛的治疗特性可能会改善相关突变的后果(氧化应激、炎症、线粒体功能障碍、坏死)。在这项研究中,我们向 22 名肌营养不良症患者施用了含有脐带间充质干细胞 (UC-MSCs) 的先进治疗药物。患者每个疗程接受一到五次静脉内和/或鞘内注射,每 2 个月最多两个疗程。使用了四种标准剂量,每次注射 10、20、30 或 40 × 10 6 UC-MSC;每公斤的近似剂量为 1 × 10 6UC-MSC。使用一组 CQ Dynamometer 计算机测力计(CQ Elektronik System,Czernica,Poland)测量肌肉力量。全组肌力统计分析显示右上肢明显改善(+4.0 N);左髋伸直(+4.5 N)和内收(+0.5 N);右髋伸直 (+1.0 N)、弯曲 (+7.5 N) 和内收 (+2.5 N);右膝拉直(+8.5 N);左肩撤销 (+13.0 N)、拉直 (+5.5 N) 和弯曲 (+6.5 N);右肩内收 (+3.0 N)、收回 (+10.5 N) 和弯曲 (+5 N);和右肘矫直(+9.5 N);所有这些差异都具有统计学意义。在 6 名患者 (27.3%) 中,这些变化导致步态分析或运动量表结果的改善。只有一名患者在最后一次给药后出现短暂的头痛和腰痛。总之,UC-MSC 治疗可被视为这些患者的治疗选择。
更新日期:2021-09-23
中文翻译:

脐带来源间充质干细胞在肌营养不良患者中的应用:在现实生活中富有同情心使用的结果
肌营养不良症是遗传决定的进行性疾病,没有病因相关的治疗和有限的支持治疗。尽管干细胞不能解决潜在的遗传条件,但它们广泛的治疗特性可能会改善相关突变的后果(氧化应激、炎症、线粒体功能障碍、坏死)。在这项研究中,我们向 22 名肌营养不良症患者施用了含有脐带间充质干细胞 (UC-MSCs) 的先进治疗药物。患者每个疗程接受一到五次静脉内和/或鞘内注射,每 2 个月最多两个疗程。使用了四种标准剂量,每次注射 10、20、30 或 40 × 10 6 UC-MSC;每公斤的近似剂量为 1 × 10 6UC-MSC。使用一组 CQ Dynamometer 计算机测力计(CQ Elektronik System,Czernica,Poland)测量肌肉力量。全组肌力统计分析显示右上肢明显改善(+4.0 N);左髋伸直(+4.5 N)和内收(+0.5 N);右髋伸直 (+1.0 N)、弯曲 (+7.5 N) 和内收 (+2.5 N);右膝拉直(+8.5 N);左肩撤销 (+13.0 N)、拉直 (+5.5 N) 和弯曲 (+6.5 N);右肩内收 (+3.0 N)、收回 (+10.5 N) 和弯曲 (+5 N);和右肘矫直(+9.5 N);所有这些差异都具有统计学意义。在 6 名患者 (27.3%) 中,这些变化导致步态分析或运动量表结果的改善。只有一名患者在最后一次给药后出现短暂的头痛和腰痛。总之,UC-MSC 治疗可被视为这些患者的治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号