当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Stereoselective 1,2-Carboboration of Ynamides with Aryldichloroboranes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-26 , DOI: 10.1002/anie.202107647 Cai You 1 , Mika Sakai 2 , Constantin G Daniliuc 1 , Klaus Bergander 1 , Shigehiro Yamaguchi 2, 3 , Armido Studer 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-26 , DOI: 10.1002/anie.202107647 Cai You 1 , Mika Sakai 2 , Constantin G Daniliuc 1 , Klaus Bergander 1 , Shigehiro Yamaguchi 2, 3 , Armido Studer 1
Affiliation
|
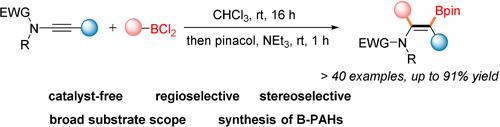
Catalyst-free 1,2-carboboration of ynamides is presented. Readily available aryldichloroboranes react with alkyl- or aryl-substituted ynamides in high yields with complete regio- and stereoselectivity to valuable β-boryl-β-alkyl/aryl α-aryl substituted enamides which belong to the class of trisubstituted alkenylboronates. The 1,2-carboboration reaction is experimentally easy to conduct, shows high functional group tolerance and broad substrate scope. Gram-scale reactions and diverse synthetic transformations convincingly demonstrate the synthetic potential of this method. The reaction can also be used to access 1-boraphenalenes, a class of boron-doped polycyclic aromatic hydrocarbons.
中文翻译:

炔烃与芳基二氯硼烷的区域选择性和立体选择性 1,2-碳硼化反应
介绍了 ynamides 的无催化剂 1,2-碳硼化。容易获得的芳基二氯硼烷与烷基或芳基取代的炔酰胺以高产率反应,对有价值的 β-硼基-β-烷基/芳基 α-芳基取代的烯酰胺具有完全的区域和立体选择性,属于三取代的烯基硼酸酯类。1,2-碳硼化反应在实验上易于进行,显示出高官能团耐受性和广泛的底物范围。克级反应和各种合成转化令人信服地证明了该方法的合成潜力。该反应还可用于获得 1-boraphenalenes,一类掺硼的多环芳烃。
更新日期:2021-09-20
中文翻译:

炔烃与芳基二氯硼烷的区域选择性和立体选择性 1,2-碳硼化反应
介绍了 ynamides 的无催化剂 1,2-碳硼化。容易获得的芳基二氯硼烷与烷基或芳基取代的炔酰胺以高产率反应,对有价值的 β-硼基-β-烷基/芳基 α-芳基取代的烯酰胺具有完全的区域和立体选择性,属于三取代的烯基硼酸酯类。1,2-碳硼化反应在实验上易于进行,显示出高官能团耐受性和广泛的底物范围。克级反应和各种合成转化令人信服地证明了该方法的合成潜力。该反应还可用于获得 1-boraphenalenes,一类掺硼的多环芳烃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号