当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequence requirements of the FFAT-like motif for specific binding to VAP-A are revealed by NMR
FEBS Letters ( IF 3.5 ) Pub Date : 2021-07-27 , DOI: 10.1002/1873-3468.14166 Kyoko Furuita 1 , Marina Hiraoka 2 , Kentaro Hanada 3 , Toshimichi Fujiwara 1 , Chojiro Kojima 1, 2
FEBS Letters ( IF 3.5 ) Pub Date : 2021-07-27 , DOI: 10.1002/1873-3468.14166 Kyoko Furuita 1 , Marina Hiraoka 2 , Kentaro Hanada 3 , Toshimichi Fujiwara 1 , Chojiro Kojima 1, 2
Affiliation
|
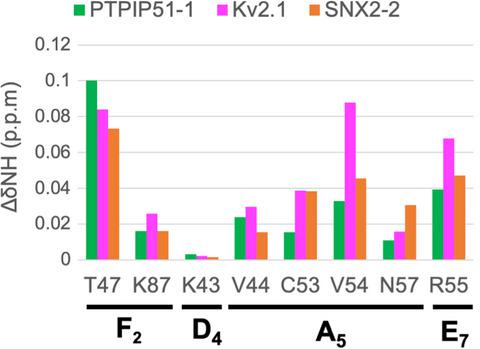
The endoplasmic reticulum transmembrane protein vesicle-associated membrane protein-associated protein (VAP) plays a central role in the formation and function of membrane contact sites (MCS) through its interactions with proteins. The major sperm protein (MSP) domain of VAP binds to a variety of sequences which are referred to as FFAT-like motifs. In this study, we investigated the interactions of eight peptides containing FFAT-like motifs with the VAP-A MSP domain (VAP-AMSP) by solution NMR. Six of eight peptides are specifically bound to VAP-A. Furthermore, we found that the RNA-dependent RNA polymerase of severe acute respiratory syndrome coronavirus 2 has an FFAT-like motif which specifically binds to VAP-AMSP as well as other FFAT-like motifs. Our results will contribute to the discovery of new VAP interactors.
中文翻译:

核磁共振揭示了 FFAT 样基序与 VAP-A 特异性结合的序列要求
内质网跨膜蛋白囊泡相关膜蛋白相关蛋白 (VAP) 通过其与蛋白质的相互作用,在膜接触位点 (MCS) 的形成和功能中起核心作用。VAP 的主要精子蛋白 (MSP) 结构域与多种序列结合,这些序列被称为 FFAT 样基序。在这项研究中,我们通过溶液核磁共振研究了含有 FFAT 样基序的八种肽与 VAP-A MSP 结构域 (VAP-A MSP )的相互作用。八种肽中有六种与 VAP-A 特异性结合。此外,我们发现严重急性呼吸综合征冠状病毒 2 的 RNA 依赖性 RNA 聚合酶具有与 VAP-A MSP特异性结合的 FFAT 样基序。以及其他类似 FFAT 的图案。我们的结果将有助于发现新的 VAP 相互作用物。
更新日期:2021-09-13
中文翻译:

核磁共振揭示了 FFAT 样基序与 VAP-A 特异性结合的序列要求
内质网跨膜蛋白囊泡相关膜蛋白相关蛋白 (VAP) 通过其与蛋白质的相互作用,在膜接触位点 (MCS) 的形成和功能中起核心作用。VAP 的主要精子蛋白 (MSP) 结构域与多种序列结合,这些序列被称为 FFAT 样基序。在这项研究中,我们通过溶液核磁共振研究了含有 FFAT 样基序的八种肽与 VAP-A MSP 结构域 (VAP-A MSP )的相互作用。八种肽中有六种与 VAP-A 特异性结合。此外,我们发现严重急性呼吸综合征冠状病毒 2 的 RNA 依赖性 RNA 聚合酶具有与 VAP-A MSP特异性结合的 FFAT 样基序。以及其他类似 FFAT 的图案。我们的结果将有助于发现新的 VAP 相互作用物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号