Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.jsb.2021.107774 Junwen Ma 1 , Yanxiao Li 1 , Susu Han 2 , Zhengqiang Jiang 2 , Qiaojuan Yan 1 , Shaoqing Yang 2
|
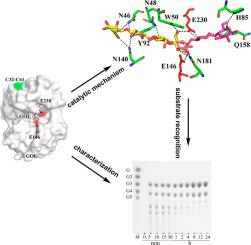
β-1,3-1,4-Glucanases are a type of hydrolytic enzymes capable of catalyzing the strict cleavage of β-1,4 glycosidic bonds adjacent to β-1,3 linkages in β-D-glucans and have exhibited great potential in food and feed industrials. In this study, a novel glycoside hydrolase (GH) family 12 β-1,3-1,4-glucanase (CtGlu12A) from the thermophilic fungus Chaetomium sp. CQ31 was identified and biochemically characterized. CtGlu12A was most active at pH 7.5 and 65 °C, respectively, and exhibited a high specific activity of 999.9 U mg−1 towards lichenin. It maintained more than 80% of its initial activity in a wide pH range of 5.0–11.0, and up to 60 °C after incubation at 55 °C for 60 min. Moreover, the crystal structures of CtGlu12A with gentiobiose and tetrasccharide were resolved. CtGlu12A had a β-jellyroll fold, and performed retaining mechanism with two glutamic acids severing as the catalytic residues. In the complex structure, cellobiose molecule showed two binding modes, occupying subsites −2 to −1 and subsites + 1 to + 2, respectively. The concave cleft made mixed β-1,3-1,4-glucan substrates maintain a bent conformation to fit into the active site. Overall, this study is not only helpful for the understanding of the substrate-binding model and catalytic mechanism of GH 12 β-1,3-1,4-glucanases, but also provides a basis for further enzymatic engineering of β-1,3-1,4-glucanases.
中文翻译:

对来自毛壳菌属的糖苷水解酶家族 12 β-1,3-1,4-葡聚糖酶的底物结合机制的结构和生化见解。
β-1,3-1,4-葡聚糖酶是一种水解酶,能够催化β-D-葡聚糖中与β-1,3键相邻的β-1,4糖苷键的严格裂解,具有很大的应用潜力在食品和饲料工业。在这项研究中,一种来自嗜热真菌毛壳菌的新型糖苷水解酶 (GH) 家族 12 β-1,3-1,4-葡聚糖酶 (CtGlu12A) 。CQ31 被鉴定和生化特征。CtGlu12A 分别在 pH 7.5 和 65 °C 时最活跃,并表现出 999.9 U mg -1的高比活性朝向地衣素。它在 5.0-11.0 的宽 pH 范围内保持超过 80% 的初始活性,在 55°C 孵育 60 分钟后最高可达 60°C。此外,还解析了具有龙胆二糖和四糖的 CtGlu12A 的晶体结构。CtGlu12A具有β-果冻折叠,并以两种谷氨酸作为催化残基进行保留机制。在复杂的结构中,纤维二糖分子表现出两种结合模式,分别占据亚位点-2至-1和亚位点+1至+2。由凹裂制成的混合 β-1,3-1,4-葡聚糖底物保持弯曲构象以适合活性位点。总体而言,本研究不仅有助于理解GH 12 β-1,3-1,4-葡聚糖酶的底物结合模型和催化机制,也为进一步酶解β-1,3提供了依据。 -1,











































 京公网安备 11010802027423号
京公网安备 11010802027423号