Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.enzmictec.2021.109884 Ling Zhang 1 , Jiaze Li 1 , Xiawen Wang 1 , Zhaoqi Ran 1 , Qi Shang 1 , Chan Chen 1 , Weikang Tang 1 , Wenbin Liu 1
|
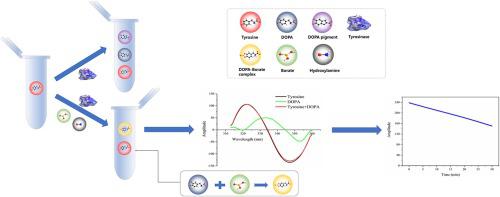
Tyrosinase plays an essential role in melanin biosynthesis and inherently exhibits both monophenolase and diphenolase activity. A first derivative synchronous fluorometric assay was established for directly monitoring monophenolase activity. The zero-crossing point at 322 nm for the first-derivative under synchronous fluorescence with Δλ = 67 nm was utilized to selectively quantify tyrosine in the presence of the reaction product dihydroxyphenylalanine (DOPA). The limit of detection (LOD) for tyrosine was 0.54 μM. The fluorescence intensity of tyrosine was monitored at intervals of 30 s to establish the time course of tyrosine consumption. The LOD for the monophenolase activity was 0.0706 U⋅ mL−1. The Michaelis-Menten e constant and maximum speed were 21.83 μM and 1.12 μM min−1, respectively. Zinc ions competitively inhibited the monophenolase activity, with an IC50 value of 14.36 μM. This assay is easily and rapidly executed and is of great significance for analyzing the kinetics of enzymatic reactions and in fundamental research on monophenolase. This approach has potential applications in the discovery of tyrosinase inhibitors for medicine and cosmetics, as well as in the industrial synthesis of substituted o-diphenol intermediates.
中文翻译:

连续测量单酚酶活性的一阶导数同步荧光法
酪氨酸酶在黑色素生物合成中起重要作用,并且固有地表现出单酚酶和二酚酶活性。建立了用于直接监测单酚酶活性的一阶导数同步荧光测定法。在Δλ = 67 nm 的同步荧光下,一阶衍生物在 322 nm 处的零交叉点用于在反应产物二羟基苯丙氨酸 (DOPA) 存在下选择性定量酪氨酸。酪氨酸的检测限 (LOD) 为 0.54 μM。以 30 秒的间隔监测酪氨酸的荧光强度,以确定酪氨酸消耗的时间过程。单酚酶活性的 LOD 为 0.0706 U⋅ mL -1。Michaelis-Menten e 常数和最大速度分别为 21.83 μM 和 1.12 μM min-1,分别。锌离子竞争性抑制单酚酶活性,IC 50值为14.36 μM。该方法操作简单、快速,对酶促反应动力学分析和单酚酶基础研究具有重要意义。该方法在发现用于医药和化妆品的酪氨酸酶抑制剂以及取代邻二酚中间体的工业合成中具有潜在的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号