Frontiers of Environmental Science & Engineering ( IF 6.1 ) Pub Date : 2021-07-27 , DOI: 10.1007/s11783-021-1489-0 Yang Yang 1 , Qi Zhang 1 , Baiyang Chen 1 , Liangchen Long 1 , Guan Zhang 1
|
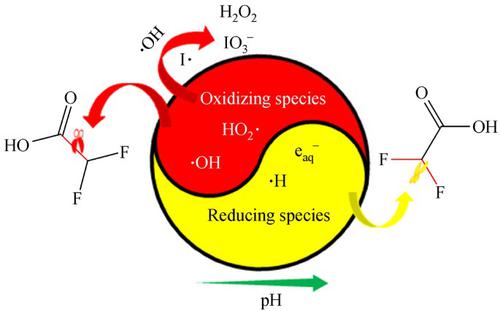
Recently, a photochemical process induced by ultraviolet (UV), vacuum UV (VUV), and iodide (I−) has gained attention for its robust potential for contaminant degradation. However, the mechanisms behind this process remain unclear because both oxidizing and reducing reactants are likely generated. To better understand this process, this study examined the evolutions of hydrogen peroxide (H2O2) and iodine species (i.e., iodide, iodate, and triiodide) during the UV/VUV/I− process under varying pH and dissolved oxygen (DO) conditions. Results show that increasing DO in water enhanced H2O2 and iodate production, suggesting that high DO favors the formation of oxidizing species. In contrast, increasing pH (from 6.0 to 11.0) resulted in lower H2O2 and iodate formation, indicating that there was a decrease of oxidative capacity for the UV/VUV/I− process. In addition, difluoroacetic acid (DFAA) was used as an exemplar contaminant to verify above observations. Although its degradation kinetics did not follow a constant trend as pH increases, the relative importance of mineralization appeared declining, suggesting that there was a redox transition from an oxidizing environment to a reducing environment as pH rises. The treatability of the UV/VUV/I− process was stronger than UV/VUV under pH of 11.0, while UV/VUV process presented a better performance at pH lower than 11.0.
中文翻译:

更好地理解真空紫外线——碘化物通过过氧化氢形成、碘种类变化和二氟乙酸降解诱导光解
最近,由紫外线 (UV)、真空紫外线 (VUV) 和碘化物 (I - )诱导的光化学过程因其强大的污染物降解潜力而受到关注。然而,该过程背后的机制仍不清楚,因为可能会产生氧化和还原反应物。为了更好地理解这一过程,本研究检查了在不同 pH 值和溶解氧 (DO) 条件下UV/VUV/I −过程中过氧化氢 (H 2 O 2 ) 和碘物质(即碘化物、碘酸盐和三碘化物)的演变) 状况。结果表明,增加水中 DO 增强了 H 2 O 2和碘酸盐的产生,表明高 DO 有利于氧化物质的形成。相比之下,增加 pH 值(从 6.0 到 11.0)导致 H 2 O 2和碘酸盐的形成降低,表明 UV/VUV/I -过程的氧化能力降低。此外,二氟乙酸 (DFAA) 被用作示例污染物以验证上述观察结果。尽管随着 pH 值的增加,其降解动力学并未遵循恒定趋势,但矿化的相对重要性似乎在下降,这表明随着 pH 值的升高,存在从氧化环境到还原环境的氧化还原转变。UV/VUV/I 的可处理性- 在pH 11.0 下,UV/VUV 工艺比UV/VUV 工艺更强,而在pH 低于11.0 时UV/VUV 工艺表现出更好的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号