Cell ( IF 64.5 ) Pub Date : 2021-07-26 , DOI: 10.1016/j.cell.2021.07.003 Kathryn R Bowles 1 , M Catarina Silva 2 , Kristen Whitney 3 , Taylor Bertucci 4 , Joshua E Berlind 5 , Jesse D Lai 6 , Jacob C Garza 2 , Nathan C Boles 4 , Sidhartha Mahali 7 , Kevin H Strang 3 , Jacob A Marsh 7 , Cynthia Chen 7 , Derian A Pugh 1 , Yiyuan Liu 1 , Ronald E Gordon 8 , Susan K Goderie 4 , Rebecca Chowdhury 4 , Steven Lotz 4 , Keith Lane 4 , John F Crary 8 , Stephen J Haggarty 2 , Celeste M Karch 7 , Justin K Ichida 5 , Alison M Goate 1 , Sally Temple 4
|
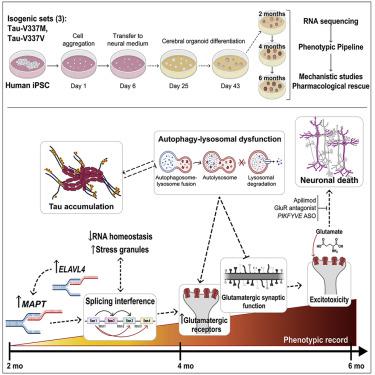
Frontotemporal dementia (FTD) because of MAPT mutation causes pathological accumulation of tau and glutamatergic cortical neuronal death by unknown mechanisms. We used human induced pluripotent stem cell (iPSC)-derived cerebral organoids expressing tau-V337M and isogenic corrected controls to discover early alterations because of the mutation that precede neurodegeneration. At 2 months, mutant organoids show upregulated expression of MAPT, glutamatergic signaling pathways, and regulators, including the RNA-binding protein ELAVL4, and increased stress granules. Over the following 4 months, mutant organoids accumulate splicing changes, disruption of autophagy function, and build-up of tau and P-tau-S396. By 6 months, tau-V337M organoids show specific loss of glutamatergic neurons as seen in individuals with FTD. Mutant neurons are susceptible to glutamate toxicity, which can be rescued pharmacologically by the PIKFYVE kinase inhibitor apilimod. Our results demonstrate a sequence of events that precede neurodegeneration, revealing molecular pathways associated with glutamate signaling as potential targets for therapeutic intervention in FTD.
中文翻译:

ELAVL4、剪接和谷氨酸能功能障碍先于 MAPT 突变脑类器官中的神经元丢失
由于MAPT突变导致的额颞叶痴呆 (FTD) 通过未知机制导致 tau 和谷氨酸能皮质神经元的病理性积累。我们使用人类诱导多能干细胞 (iPSC) 衍生的表达 tau-V337M 的脑类器官和等基因校正对照来发现由于神经变性之前的突变而导致的早期改变。在 2 个月时,突变类器官表现出MAPT、谷氨酸能信号通路和调节剂(包括 RNA 结合蛋白ELAVL4 )的上调表达, 和增加的应力颗粒。在接下来的 4 个月中,突变类器官积累了剪接变化、自噬功能破坏以及 tau 和 P-tau-S396 的积累。到 6 个月时,tau-V337M 类器官表现出特定的谷氨酸能神经元丢失,这在 FTD 患者中可见。突变神经元易受谷氨酸毒性的影响,PIKFYVE 激酶抑制剂阿匹莫德可以在药理学上挽救这种毒性。我们的研究结果证明了神经退行性变之前的一系列事件,揭示了与谷氨酸信号传导相关的分子途径作为 FTD 治疗干预的潜在目标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号