当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Photoelectrochemical Conversion of Ammonium to Dinitrogen Using a Bromide-Mediated Redox Cycle
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-07-26 , DOI: 10.1021/acsestengg.1c00119 Min Seok Koo 1 , Seungmok Han 1 , Kangwoo Cho 1 , Wonyong Choi 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-07-26 , DOI: 10.1021/acsestengg.1c00119 Min Seok Koo 1 , Seungmok Han 1 , Kangwoo Cho 1 , Wonyong Choi 1
Affiliation
|
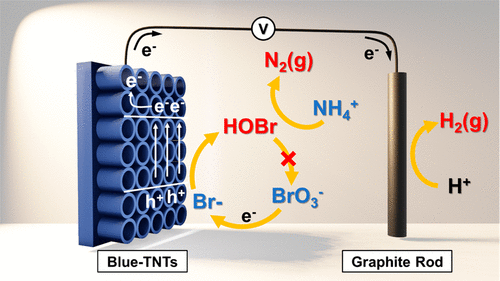
Bromide ion (Br–) can be oxidized to reactive bromine species (RBS; Br•, Br2•–, and HOBr/OBr–) which can serve as an effective alternative to chlorine disinfectant. This study investigated the generation of RBS in a photoelectrochemical (PEC) system using an electrochromic TiO2 nanotube arrays (Blue-TNTs) electrode under UV light (λ > 320 nm) and demonstrated the effect of RBS on the direct conversion of ammonium (NH4+) to dinitrogen (N2) with near 100% efficiency. The PEC system utilizing in situ generated RBS not only removed NH4+ more efficiently than photocatalytic (PC) and electrochemical (EC) systems but also prevented the generation of unwanted products (i.e., NO2– and NO3–). In addition, compared with the PEC-Cl system, the PEC-Br system exhibited a superior ammonium removal efficiency (16% vs 95% for 120 min of reaction; under air-equilibrated condition). The PEC system also showed higher NH4+ removal efficiency and lower energy consumption when compared to an EC system (using a boron-doped diamond electrode). While bromate ions (BrO3–) are produced as a toxic byproduct of bromide oxidation in a typical ozonation system, the Blue-TNTs PEC-Br system fully hinders the bromate formation as long as ammonium is present in the solution because RBS rapidly reacts with NH4+ with little chance of further oxidation to bromate.
中文翻译:

使用溴化物介导的氧化还原循环将铵定量光电化学转化为二氮
溴离子 (Br – ) 可被氧化成活性溴物质(RBS;Br •、Br 2 •–和 HOBr/OBr –),可作为氯消毒剂的有效替代品。本研究调查了在紫外光 (λ > 320 nm) 下使用电致变色 TiO 2纳米管阵列 (Blue-TNTs) 电极在光电化学 (PEC) 系统中生成 RBS,并证明了 RBS 对铵 (NH 4 + ) 转化为二氮 (N 2 ),效率接近 100%。利用原位生成的 RBS 的 PEC 系统不仅去除了 NH 4 +比光催化 (PC) 和电化学 (EC) 系统更有效,而且还能防止产生不需要的产物(即 NO 2 –和 NO 3 –)。此外,与 PEC-Cl 系统相比,PEC-Br 系统表现出优异的铵去除效率(16% vs 95%,反应 120 分钟;在空气平衡条件下)。与 EC 系统(使用掺硼金刚石电极)相比,PEC 系统还显示出更高的 NH 4 +去除效率和更低的能耗。而溴酸根离子 (BrO 3 –) 在典型的臭氧化系统中作为溴化物氧化的有毒副产物产生,只要溶液中存在铵,Blue-TNTs PEC-Br 系统就会完全阻碍溴酸盐的形成,因为 RBS 与 NH 4 +迅速反应,几乎没有机会进一步氧化成溴酸盐。
更新日期:2021-09-10
中文翻译:

使用溴化物介导的氧化还原循环将铵定量光电化学转化为二氮
溴离子 (Br – ) 可被氧化成活性溴物质(RBS;Br •、Br 2 •–和 HOBr/OBr –),可作为氯消毒剂的有效替代品。本研究调查了在紫外光 (λ > 320 nm) 下使用电致变色 TiO 2纳米管阵列 (Blue-TNTs) 电极在光电化学 (PEC) 系统中生成 RBS,并证明了 RBS 对铵 (NH 4 + ) 转化为二氮 (N 2 ),效率接近 100%。利用原位生成的 RBS 的 PEC 系统不仅去除了 NH 4 +比光催化 (PC) 和电化学 (EC) 系统更有效,而且还能防止产生不需要的产物(即 NO 2 –和 NO 3 –)。此外,与 PEC-Cl 系统相比,PEC-Br 系统表现出优异的铵去除效率(16% vs 95%,反应 120 分钟;在空气平衡条件下)。与 EC 系统(使用掺硼金刚石电极)相比,PEC 系统还显示出更高的 NH 4 +去除效率和更低的能耗。而溴酸根离子 (BrO 3 –) 在典型的臭氧化系统中作为溴化物氧化的有毒副产物产生,只要溶液中存在铵,Blue-TNTs PEC-Br 系统就会完全阻碍溴酸盐的形成,因为 RBS 与 NH 4 +迅速反应,几乎没有机会进一步氧化成溴酸盐。











































 京公网安备 11010802027423号
京公网安备 11010802027423号