Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-07-24 , DOI: 10.1016/j.jcou.2021.101644 Zhaoyang Wang 1, 2 , Ruijun Xie 1, 2 , Hailong Hong 1, 2 , Limin Han 1, 2, 3 , Ning Zhu 1, 2
|
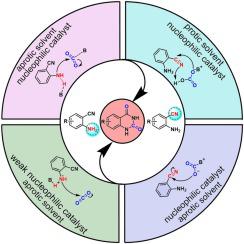
Quinazoline-2,4(1H,3H)-dione derivatives are key intermediates in pharmaceutical, biological and chemical fields. To avoid the use of toxic carbonylation reagents such as phosgene, carbon monoxide and potassium cyanate, green synthesis methods for preparing quinazolindiones via the reaction of o-aminobenzonitrile with CO2 are developed. In this review, the factors such as reaction medium, catalyst and reaction temperature that influence the reaction of o-aminobenzonitrile with CO2 are systematically discussed. The protic solvent is favorable for the nitrile group as the initial reaction site to react with CO2. In contrast, the reactivity of the nitrile group and amino group of o-aminobenzonitrile could hardly be affected by aprotic solvent. Moreover, the reaction is also strongly affected by the catalyst, which is evaluated by the basicity and nucleophilicity of the catalyst. Specifically, the basicity mainly affects the activation of the amino group in the o-aminobenzonitrile substrate, whereas the nucleophilicity primarily impacts the formation of the CO2 activated state. The similarities and differences of different catalysts such as organic bases, inorganic bases, ionic liquids and supported catalysts in specific reaction pathways are also discussed in this review. In addition, the formation of isocyanate intermediate in the rearrangement process is significantly affected by the reaction temperature. The quinazolindione could be easily formed from the isocyanate intermediate, isomerized from the cyclic carbamate, under high reaction temperature. Furthermore, the quinazolindione could be formed directly by the isomerized cyclic carbamate under low reaction temperature.
中文翻译:

CO 2与邻氨基苄腈合成喹唑啉-2,4-二酮的机理和反应条件
喹唑啉-2,4(1H,3H)-二酮衍生物是制药、生物和化学领域的关键中间体。为避免使用光气、一氧化碳和氰酸钾等有毒羰基化试剂,开发了通过邻氨基苯甲腈与CO 2反应制备喹唑啉二酮的绿色合成方法。本综述系统地讨论了影响邻氨基苄腈与CO 2反应的反应介质、催化剂和反应温度等因素。质子溶剂有利于腈基作为初始反应位点与 CO 2反应. 相比之下,邻氨基苄腈的腈基和氨基的反应性几乎不受非质子溶剂的影响。此外,反应也受催化剂的强烈影响,这是通过催化剂的碱性和亲核性来评价的。具体而言,碱性主要影响邻氨基苯甲腈底物中氨基的活化,而亲核性主要影响 CO 2的形成激活状态。本综述还讨论了有机碱、无机碱、离子液体和负载型催化剂等不同催化剂在特定反应途径中的异同。此外,重排过程中异氰酸酯中间体的形成受反应温度的影响很大。在高反应温度下,由环状氨基甲酸酯异构化的异氰酸酯中间体很容易形成喹唑啉二酮。此外,异构化的环状氨基甲酸酯可以在较低的反应温度下直接形成喹唑啉二酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号