当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The structural, functional, and dynamic effect of Tau tubulin kinase1 upon a mutation: A neuro-degenerative hotspot
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-07-23 , DOI: 10.1002/jcb.30112 Shahzaib Ahamad 1 , Kanipakam Hema 1 , Vijay Kumar 2 , Dinesh Gupta 1
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-07-23 , DOI: 10.1002/jcb.30112 Shahzaib Ahamad 1 , Kanipakam Hema 1 , Vijay Kumar 2 , Dinesh Gupta 1
Affiliation
|
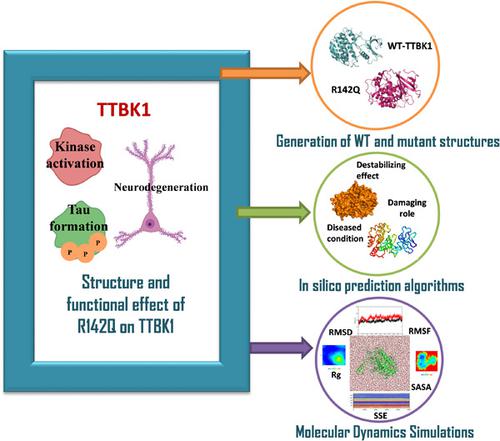
Alzheimer's disease (AD) is a progressive disorder that causes brain cells to degenerate and die. AD is one of the common causes of dementia that leads to a decline in thinking, behavioral and social skills that disrupts a person's ability to function independently. Tau-tubulin kinase1 (TTBK1) is a crucial disease regulating AD protein, which is majorly responsible for the phosphorylation and accumulation of tau protein at specific Serine/Threonine residues found in paired helical filaments, suggesting its role in tauopathy. TTBK1 involvement in many diseases and the restricted expression of TTBK1 to the central nervous system (CNS) makes TTBK1 an attractive therapeutic target for tauopathies. The genetic variations in TTBK1 are primarily involved in the TTBK1 pathogenesis. This study highlighted the destabilizing, damaging and deleterious effect of the mutation R142Q on TTBK1 structure through computational predictions and molecular dynamics simulations. The protein deviation, fluctuations, conformational dynamics, solvent accessibility, hydrogen bonding, and the residue-residue mapping confirmed the mutant effect to cause structural aberrations, suggesting overall destabilization due to the protein mutation. The presence of well-defined free energy minima was observed in TTBK1-wild type, as opposed to that in the R142Q mutant, reflecting structural deterioration. The overall findings from the study reveal that the presence of R142Q mutation on TTBK1 is responsible for the structural instability, leading to disruption of its biological functions. The mutation could be used as future diagnostic markers in treating AD.
中文翻译:

Tau 微管蛋白激酶 1 对突变的结构、功能和动态影响:一个神经退行性热点
阿尔茨海默病 (AD) 是一种进行性疾病,会导致脑细胞退化和死亡。AD 是痴呆症的常见原因之一,它会导致思维、行为和社交技能下降,从而破坏一个人独立运作的能力。Tau-微管蛋白激酶1 ( TTBK1 ) 是一种调节 AD 蛋白的关键疾病,它主要负责 tau 蛋白在成对螺旋丝中发现的特定丝氨酸/苏氨酸残基处的磷酸化和积累,表明其在 tau 病变中的作用。TTBK1参与多种疾病,TTBK1对中枢神经系统 (CNS) 的限制性表达使得TTBK1tau蛋白病的一个有吸引力的治疗靶点。TTBK1的遗传变异主要参与TTBK1的发病机制。本研究通过计算预测和分子动力学模拟强调了突变 R142Q 对TTBK1结构的不稳定、破坏和有害影响。蛋白质偏差、波动、构象动力学、溶剂可及性、氢键和残基-残基作图证实了突变效应导致结构异常,表明蛋白质突变导致整体不稳定。在TTBK1中观察到明确定义的自由能最小值的存在-野生型,与 R142Q 突变体相反,反映结构恶化。该研究的总体结果表明,TTBK1 上 R142Q 突变的存在是造成结构不稳定的原因,从而导致其生物学功能的破坏。该突变可用作治疗AD的未来诊断标志物。
更新日期:2021-07-23
中文翻译:

Tau 微管蛋白激酶 1 对突变的结构、功能和动态影响:一个神经退行性热点
阿尔茨海默病 (AD) 是一种进行性疾病,会导致脑细胞退化和死亡。AD 是痴呆症的常见原因之一,它会导致思维、行为和社交技能下降,从而破坏一个人独立运作的能力。Tau-微管蛋白激酶1 ( TTBK1 ) 是一种调节 AD 蛋白的关键疾病,它主要负责 tau 蛋白在成对螺旋丝中发现的特定丝氨酸/苏氨酸残基处的磷酸化和积累,表明其在 tau 病变中的作用。TTBK1参与多种疾病,TTBK1对中枢神经系统 (CNS) 的限制性表达使得TTBK1tau蛋白病的一个有吸引力的治疗靶点。TTBK1的遗传变异主要参与TTBK1的发病机制。本研究通过计算预测和分子动力学模拟强调了突变 R142Q 对TTBK1结构的不稳定、破坏和有害影响。蛋白质偏差、波动、构象动力学、溶剂可及性、氢键和残基-残基作图证实了突变效应导致结构异常,表明蛋白质突变导致整体不稳定。在TTBK1中观察到明确定义的自由能最小值的存在-野生型,与 R142Q 突变体相反,反映结构恶化。该研究的总体结果表明,TTBK1 上 R142Q 突变的存在是造成结构不稳定的原因,从而导致其生物学功能的破坏。该突变可用作治疗AD的未来诊断标志物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号