Calphad ( IF 2.4 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.calphad.2021.102318 Erting Dong 1, 2 , Shihua Tan 3 , Jiong Wang 4 , Weishu Liu 5 , Wenqing Zhang 2, 6
|
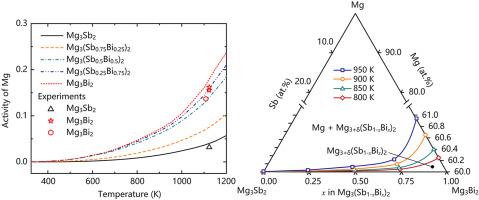
Assessment of the thermodynamic activity and solid solubility for solute element in multi-component alloy is critical in thermodynamics, but an effective first-principles estimation of the parameter has been an unsolved issue for many years. By taking the excess Mg in Mg3(Sb1-xBix)2 alloy as an example, Mg atoms could occupy the regular lattice sites and the interstitial sites as well, which makes the estimation of thermodynamic activity of Mg solute not easy. In this work, we develop a model to estimate the activity and solid solubility of Mg in alloys such as Mg3(Sb1-xBix)2 by considering the formation energy contribution from both Mg interstitials and Mg vacancies, and the associated entropies to final Gibbs energy. Accurate assessment of the parameter by using first-principles thermodynamics approach is also realized. The estimated activity of Mg in Mg3Bi2 and Mg3Sb2 reproduce well the available experimental results. The solubility limits of Mg and equilibrium concentrations of Mg interstitials and Mg vacancies in Mg3(Sb1-xBix)2 are also estimated. The results could be used to understand Mg–Mg3Sb2–Mg3Bi2 phase diagram as well as the Mg effect on thermoelectric performance of Mg3(Sb1-xBix)2 alloy.
中文翻译:

从第一性原理看多组分合金中溶质的热力学活性:以Mg 3 (Sb 1- x Bi x ) 2 中过量Mg为例
评估多组分合金中溶质元素的热力学活性和固溶度在热力学中是至关重要的,但多年来对该参数的有效第一性原理估计一直是一个悬而未决的问题。以Mg 3 (Sb 1- x Bi x ) 2合金中过量的Mg为例,Mg原子既可以占据规则的晶格位置,也可以占据间隙位置,这使得对Mg溶质的热力学活性的估计并不容易。在这项工作中,我们开发了一个模型来估计镁在合金中的活性和固溶度,例如 Mg 3 (Sb 1- x Bi x ) 2通过考虑 Mg 间隙和 Mg 空位的形成能贡献,以及相关的熵对最终吉布斯能量的影响。还实现了使用第一性原理热力学方法对参数的准确评估。Mg 3 Bi 2和Mg 3 Sb 2中Mg的估计活性很好地再现了可用的实验结果。还估计了 Mg 的溶解度极限以及 Mg 3 (Sb 1- x Bi x ) 2中Mg 间隙和 Mg 空位的平衡浓度。结果可用于理解 Mg-Mg 3 Sb 2 -Mg 3 Bi图2相图以及Mg对Mg 3 (Sb 1- x Bi x ) 2合金热电性能的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号