Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-07-23 , DOI: 10.1016/j.seppur.2021.119351 Xiaowei Cui 1, 2 , Shuo-Shuo Zhang 2 , Yong Geng 1 , Jianyuan Zhen 2 , Jinhua Zhan 3 , Chengbo Cao 3 , Shou-Qing Ni 2
|
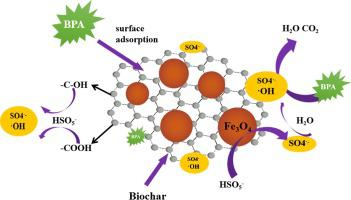
The nanoFe3O4-biochar (Fe3O4-BC) heterogeneous catalyst was synthesized by coprecipitation method and the mechanism of Fe3O4-BC/peroxymonosulfate (Fe3O4-BC/PMS) system adsorption and synergistic catalysis for the removal of bisphenol A (BPA) were studied. The effect of initial pH, Fe3O4-BC load, PMS concentration on the catalyst activity were systematically evaluated. The results showed the synergistic effect between BC and Fe3O4 can greatly improve the removal efficiency of BPA in the Fe3O4-BC/PMS system. BPA removal efficiency gained 100% in 90 min at pH 3.0 with PMS 5 mM, Fe3O4-BC load 2.0 g/L and BPA 20 mg/L. Sulfate radical (SO4•−) and hydroxyl radicals (•OH) was determined as the reactive species in the Fe3O4-BC/PMS system, and SO4•− was the major reactive species. Fe3O4-BC remained high active and the iron loss was only 2.13 mg/L when the pH was 3.0 after five recycles, which indicates that Fe3O4-BC catalyst has a good reusability potential and practical applications.
中文翻译:

Fe3O4-生物炭/过硫酸盐体系协同催化去除双酚a
所述nanoFe 3 Ô 4 -biochar(FE 3 ö 4 -BC)的非均相催化剂是通过共沉淀法和Fe的机理合成的3 ö 4 -BC /过氧单(铁3 ö 4 -BC / PMS)系统吸附和用于协同催化对双酚 A (BPA) 的去除进行了研究。系统地评估了初始 pH、Fe 3 O 4 -BC 负载、PMS 浓度对催化剂活性的影响。结果表明BC和Fe之间的协同效应3 ö 4可以大大提高BPA的在Fe的去除效率3 ö 4-BC/PMS 系统。在 PMS 5 mM、Fe 3 O 4 -BC 负载 2.0 g/L 和 BPA 20 mg/L 条件下,在 pH 3.0 条件下,BPA 去除效率在 90 分钟内提高了 100% 。硫酸根 (SO 4 •- ) 和羟基自由基 ( • OH) 被确定为 Fe 3 O 4 -BC/PMS 系统中的反应物种,而 SO 4 •-是主要的反应物种。Fe 3 O 4 -BC催化剂在 pH 3.0 循环 5 次后仍保持高活性,铁损仅为 2.13 mg/L,表明 Fe 3 O 4 -BC 催化剂具有良好的重复利用潜力和实际应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号