当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study on Geometry and Intramolecular Hydrogen Bonding in 8-Hydroxy-1-Naphthaldehyde
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-07-22 , DOI: 10.1002/bkcs.12367 Ali A. El‐Rayyes 1 , Yunusa Umar 2
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-07-22 , DOI: 10.1002/bkcs.12367 Ali A. El‐Rayyes 1 , Yunusa Umar 2
Affiliation
|
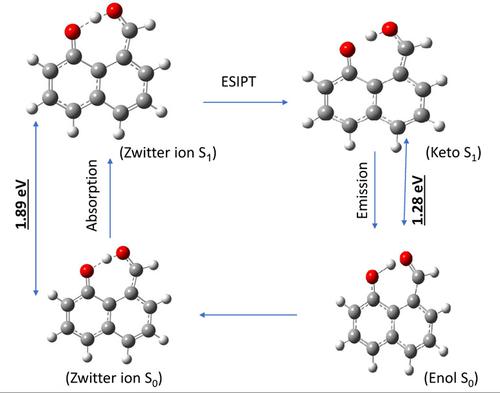
Calculated geometrical parameters in the gas phase and solvents for 8-hydroxy-1-naphthaldehyde (8H1N) were carried out using density functional theory (DFT) at the 6-311++G(d,p) level, and the Time Dependent-DFT/6-311++G(d,p) method was used for the excited state calculations. 8H1N is found to exist in two main conforms, the cis-cis conformer (where the dihedral angels C3C2-OH is 0°, C3C8-CO is 0°) was found to be more stable than the trans-trans form (where the dihedral angels C3C2-OH is 180°, C3C8 -CO is 180.0°) by about 6.47 kcal/mol. The possibility of intramolecular hydrogen bonding between the hydroxyl (OH) and aldehyde (CHO) moieties is predicted. The hydrogen bond becomes stronger in polar solvents and in the excited state. Energies of 8H1N-one water molecule complexes are calculated using the DFT-D3 level of theory and results indicate that at least one solvent molecule is required to assist the proton transfer process.
中文翻译:

8-羟基-1-萘醛的几何结构和分子内氢键的理论研究
使用密度泛函理论 (DFT) 在 6-311++G(d,p) 水平上计算 8-羟基-1-萘醛 (8H1N) 的气相和溶剂中的几何参数,以及时间相关的DFT/6-311++G(d,p) 方法用于激发态计算。发现 8H1N 存在于两种主要构象中,发现cis - cis构象异构体(其中二面角 C 3 C 2 -OH 为 0°,C 3 C 8 -CO 为 0°)比反式更稳定-反式(其中二面角 C 3 C 2 -OH 为 180°,C 3 C 8-CO 为 180.0°)约 6.47 kcal/mol。预测了羟基 (OH) 和醛 (CHO) 部分之间存在分子内氢键的可能性。氢键在极性溶剂和激发态中变得更强。8H1N-one 水分子复合物的能量是使用 DFT-D3 理论水平计算的,结果表明至少需要一种溶剂分子来协助质子转移过程。
更新日期:2021-07-22
中文翻译:

8-羟基-1-萘醛的几何结构和分子内氢键的理论研究
使用密度泛函理论 (DFT) 在 6-311++G(d,p) 水平上计算 8-羟基-1-萘醛 (8H1N) 的气相和溶剂中的几何参数,以及时间相关的DFT/6-311++G(d,p) 方法用于激发态计算。发现 8H1N 存在于两种主要构象中,发现cis - cis构象异构体(其中二面角 C 3 C 2 -OH 为 0°,C 3 C 8 -CO 为 0°)比反式更稳定-反式(其中二面角 C 3 C 2 -OH 为 180°,C 3 C 8-CO 为 180.0°)约 6.47 kcal/mol。预测了羟基 (OH) 和醛 (CHO) 部分之间存在分子内氢键的可能性。氢键在极性溶剂和激发态中变得更强。8H1N-one 水分子复合物的能量是使用 DFT-D3 理论水平计算的,结果表明至少需要一种溶剂分子来协助质子转移过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号