当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Mediated Nitrogen-Centered Radical Strategy: Preparation of 3-Acylated Spiro[4,5]trienones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-22 , DOI: 10.1002/adsc.202100852 Pu Chen 1 , Jun Xie 1 , Zan Chen 1 , Bi‐Quan Xiong 1 , Yu Liu 1 , Chang‐An Yang 1 , Ke‐Wen Tang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-22 , DOI: 10.1002/adsc.202100852 Pu Chen 1 , Jun Xie 1 , Zan Chen 1 , Bi‐Quan Xiong 1 , Yu Liu 1 , Chang‐An Yang 1 , Ke‐Wen Tang 1
Affiliation
|
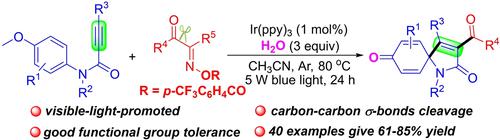
A nitrogen-centered radical strategy for the preparation of 3-acylated spiro[4,5]trienones via visible-light-mediated acylation/ipso-cyclization of alkynes with acyl oxime esters is reported. The alkyl- and aryl-substituted acyl radicals, which generate from the cleavage of carbon-carbon σ-bonds in acyl oxime esters via nitrogen-centered radical pathway, attack the carbon-carbon triple bonds in propiolamides and then undergo ipso-cyclization. This method provides a way for the construction of 3-acyl-substituted spiro[4,5]trienones, which can introduce aryl- or alkyl-substituted acyl into spiro[4,5]trienone skeletons.
中文翻译:

可见光介导的以氮为中心的自由基策略:3-酰化螺[4,5]三烯酮的制备
为3-酰化螺的制备氮为中心的自由基的策略[4,5] trienones经由可见光介导的酰化/本位与酰基肟酯炔-cyclization报道。所述烷基和芳基取代的酰基的基团,其由碳-碳裂解生成σ -键在酰基肟酯经由氮为中心的自由基途径,攻击在propiolamides碳-碳三键,并且然后经受本位-cyclization。该方法为构建3-酰基取代的螺[4,5]三烯酮提供了一种方法,可以将芳基或烷基取代的酰基引入螺[4,5]三烯酮骨架中。
更新日期:2021-09-21
中文翻译:

可见光介导的以氮为中心的自由基策略:3-酰化螺[4,5]三烯酮的制备
为3-酰化螺的制备氮为中心的自由基的策略[4,5] trienones经由可见光介导的酰化/本位与酰基肟酯炔-cyclization报道。所述烷基和芳基取代的酰基的基团,其由碳-碳裂解生成σ -键在酰基肟酯经由氮为中心的自由基途径,攻击在propiolamides碳-碳三键,并且然后经受本位-cyclization。该方法为构建3-酰基取代的螺[4,5]三烯酮提供了一种方法,可以将芳基或烷基取代的酰基引入螺[4,5]三烯酮骨架中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号