Journal of Cleaner Production ( IF 11.1 ) Pub Date : 2021-07-23 , DOI: 10.1016/j.jclepro.2021.128412 Shiquan Sun 1, 2 , Xin Zeng 1, 2 , Yang Gao 1, 2 , Wei Zhang 1, 2 , Lean Zhou 1, 2 , Xiaokang Zeng 1, 2 , Wang Liu 1, 2 , Qian Jiang 1, 2 , Changbo Jiang 1, 2 , Sixin Wang 1, 2
|
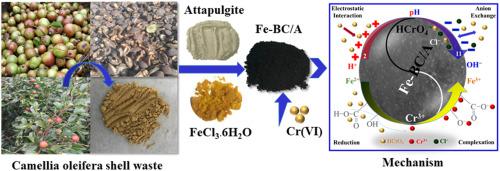
Agricultural processing by-product have been used as the feed stock for effluent treatment to promote the utilization of agricultural waste, the development of green sustainable and construct a green ecological circle nowadays. Based on camellia oleifera shells, attapulgite, and ferric chloride, a novel iron oxide loaded biochar/attapulgite composite (Fe-BC/A) was synthesized via a one-step pyrolysis process and as a low-priced and efficient adsorbent for Cr(VI) removal from aqueous solution. The structure and composition of Fe-BC/A were characterized by SEM-EDS, FT-IR, XRD, and XPS, which confirmed iron oxide (analogous to α-Fe2O3 and β-FeOOH) and attapulgite clay were successfully incorporated with biochar. The maximum adsorption capacity of Cr(VI) by Fe-BC/A was 107 mg/g at pH 2.0, which was much higher than the original biochars (BC: 54.38 mg/g) and biochar/attapulgite composites (BC/A: 81.75 mg/g). The adsorption behavior of Fe-BC/A fitted better with pseudo second-order model and Freundlich model, which indicated that multilayer chemisorption might dominate Cr(VI) adsorption. The thermodynamic analysis of Fe-BC/A represented that removal of Cr(VI) was a spontaneous and endothermic process. Batch experiments results demonstrated that Fe-BC/A had great adsorption capability of Cr(VI) in a broad pH extent of 2.0–10.0, whose most favorable elimination for Cr(VI) at pH of 2.0 and the removal efficiency of Cr(VI) was about 80% at pH = 10 (C0 = 100 mg/L, 30 °C). Fe-BC/A exhibited a prominent tolerance to the coexisting ions during Cr(VI) removal and only a slight decrease (8.27%) in elimination efficiency after five adsorption-desorption cycles. Mechanism study revealed that electrostatic attraction, reduction, anion exchange, and complexation were involved in the removal of Cr(VI). This study implied the potential practical application of Fe-BC/A in the future Cr(VI) remediation from wastewater.
中文翻译:

氧化铁负载生物炭/凹凸棒石复合材料衍生的油茶壳作为高效去除Cr(VI)的新型生物吸附剂
如今,农业加工副产品已被用作污水处理的原料,以促进农业废弃物的利用,发展绿色可持续发展,构建绿色生态圈。以油茶壳、凹凸棒石和氯化铁为基础,通过一步热解工艺合成了一种新型负载氧化铁的生物炭/凹凸棒石复合材料(Fe-BC/A),并作为一种廉价且高效的 Cr(VI) 吸附剂) 从水溶液中去除。Fe-BC/A 的结构和组成通过 SEM-EDS、FT-IR、XRD 和 XPS 表征,证实了氧化铁(类似于 α-Fe 2 O 3和 β-FeOOH) 和凹凸棒粘土成功地与生物炭结合。Fe-BC/A 对 Cr(VI) 的最大吸附容量在 pH 2.0 时为 107 mg/g,远高于原始生物炭(BC:54.38 mg/g)和生物炭/凹凸棒石复合材料(BC/A: 81.75 毫克/克)。Fe-BC/A 的吸附行为更符合伪二阶模型和 Freundlich 模型,表明多层化学吸附可能主导 Cr(VI) 吸附。Fe-BC/A 的热力学分析表明 Cr(VI) 的去除是一个自发的吸热过程。批量实验结果表明,Fe-BC/A 在 2.0-10.0 的宽 pH 范围内对 Cr(VI) 具有很好的吸附能力,在 pH 2.0 时对 Cr(VI) 的去除和 Cr(VI) 的去除效率最) 在 pH = 10 ( C0 = 100 毫克/升,30 °C)。Fe-BC/A 在 Cr(VI) 去除过程中对共存离子表现出显着的耐受性,并且在五个吸附-解吸循环后去除效率仅略有下降 (8.27%)。机理研究表明,静电吸引、还原、阴离子交换和络合参与了 Cr(VI) 的去除。该研究暗示了 Fe-BC/A 在未来废水中 Cr(VI) 修复中的潜在实际应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号