Water Research ( IF 11.4 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.watres.2021.117451 Yangju Li 1 , Haoran Dong 1 , Long Li 1 , Junyang Xiao 1 , Shuangjie Xiao 1 , Zilan Jin 1
|
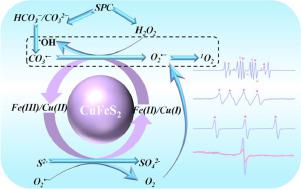
In this work, the novel application of chalcopyrite (CuFeS2) for sodium percarbonate (SPC) activation towards sulfamethazine (SMT) degradation was explored. Several key influencing factors like SPC concentration, CuFeS2 dosage, reaction temperature, pH value, anions, and humic acid (HA) were investigated. Experimental results indicated that SMT could be effectively degraded in the neutral reaction media by CuFeS2/SPC process (86.4%, 0.054 min−1 at pH = 7.1). The mechanism of SPC activation by CuFeS2 was elucidated, which was discovered to be a multiple reactive oxygen species (multi-ROS) process with the coexistence of hydroxyl radical (•OH), carbonate radical (CO3•−), superoxide radical (O2•−), and singlet oxygen (1O2), as evidenced by quenching experiments and electron spin resonance (ESR) tests. The generated •OH via the traditional heterogeneous Fenton-like process would not only react with carbonate ions to yield other ROS but also involve in SMT degradation. The abundant surface-bound Fe(II) was deemed to be the dominant catalytic active sites for SPC activation. Meanwhile, it was verified that the reductive sulfur species, the interaction between Cu(I) and Fe(III) as well as the available O2•− derived from the activation of molecular oxygen and the conversion of •OH favored the regeneration of Fe(II) on CuFeS2 surface. Furthermore, the degradation intermediates of SMT and their toxicities were evaluated. This study presents a novel strategy by integrating transition metal sulfides with percarbonate for antibiotic-contaminated water treatment.
中文翻译:

黄铜矿活化过碳酸盐有效降解磺胺二甲嘧啶
在这项工作中,探索了黄铜矿 (CuFeS 2 ) 在过碳酸钠 (SPC) 活化中对磺胺二甲嘧啶 (SMT) 降解的新应用。研究了SPC浓度、CuFeS 2用量、反应温度、pH值、阴离子和腐殖酸(HA)等几个关键影响因素。实验结果表明,通过CuFeS 2 /SPC 工艺可以在中性反应介质中有效降解SMT (86.4%,0.054 min -1,pH = 7.1)。阐明了CuFeS 2对SPC 活化的机理,发现其是一个多活性氧(multi-ROS)过程,羟基自由基(• OH)、碳酸盐自由基(CO 3•− )、超氧自由基 (O 2 •− ) 和单线态氧 ( 1 O 2 ),如淬火实验和电子自旋共振 (ESR) 测试所证明的那样。通过传统的非均质类芬顿过程生成的• OH 不仅会与碳酸根离子反应生成其他 ROS,还会参与 SMT 降解。丰富的表面结合 Fe(II) 被认为是 SPC 活化的主要催化活性位点。同时,验证了还原性硫物种、Cu(I) 和 Fe(III) 之间的相互作用以及可利用的 O 2 •-源自分子氧的活化和•OH 有利于 Fe(II) 在 CuFeS 2表面的再生。此外,还评估了 SMT 的降解中间体及其毒性。这项研究提出了一种通过将过渡金属硫化物与过碳酸盐结合来处理抗生素污染水的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号