当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
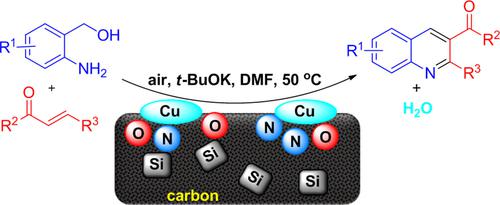
Cascade Reaction of α, β-Unsaturated Ketones and 2-Aminoaryl Alcohols for the Synthesis of 3-Acylquinolines by a Copper Nanocatalyst
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-21 , DOI: 10.1002/adsc.202100631 Yuan Liu 1 , Chen Wang 1 , Yixin Tong 1 , Yong Ling 1 , Changjian Zhou 2 , Biao Xiong 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-21 , DOI: 10.1002/adsc.202100631 Yuan Liu 1 , Chen Wang 1 , Yixin Tong 1 , Yong Ling 1 , Changjian Zhou 2 , Biao Xiong 1
Affiliation
|
3-Acylquinolines possess widespread applications in functional chemicals. However, the convenient and selective synthesis of such important substructures has to date remained a challenge. Herein, we report a method to access 3-acylquinolines from α, β-unsaturated ketones and 2-aminoaryl alcohols in one pot with a copper nanocatalyst supported on nitrogen-silica-doped carbon (Cu/N−SiO2−C). Mechanistically, the construction of the product involves a cascade procedure including radical-type oxidation of 2-aminoaryl alcohols, aza-Michael addition and annulation. This developed protocol proceeds with merits of mild reaction conditions, good functional group tolerance, earth-abundant and reusable copper catalyst, easily available stocks and O2 as the sole oxidant, which provides an alternative way for the sustainable synthesis of quinoline derivatives.
中文翻译:

α, β-不饱和酮和 2-氨基芳醇的级联反应用于铜纳米催化剂合成 3-酰基喹啉
3-酰基喹啉在功能化学品中有着广泛的应用。然而,迄今为止,这种重要子结构的方便和选择性合成仍然是一个挑战。在此,我们报告了一种在一个锅中使用负载在氮-二氧化硅掺杂碳 (Cu/N-SiO 2 -C)上的铜纳米催化剂从 α, β-不饱和酮和 2-氨基芳基醇中获得 3-酰基喹啉的方法。从机制上讲,该产品的构建涉及级联程序,包括 2-氨基芳醇的自由基型氧化、氮杂-迈克尔加成和环化。该开发的方案具有反应条件温和、官能团耐受性好、铜催化剂丰富且可重复使用、易于获得的库存和 O 2 等优点。 作为唯一的氧化剂,为喹啉衍生物的可持续合成提供了另一种途径。
更新日期:2021-09-21
中文翻译:

α, β-不饱和酮和 2-氨基芳醇的级联反应用于铜纳米催化剂合成 3-酰基喹啉
3-酰基喹啉在功能化学品中有着广泛的应用。然而,迄今为止,这种重要子结构的方便和选择性合成仍然是一个挑战。在此,我们报告了一种在一个锅中使用负载在氮-二氧化硅掺杂碳 (Cu/N-SiO 2 -C)上的铜纳米催化剂从 α, β-不饱和酮和 2-氨基芳基醇中获得 3-酰基喹啉的方法。从机制上讲,该产品的构建涉及级联程序,包括 2-氨基芳醇的自由基型氧化、氮杂-迈克尔加成和环化。该开发的方案具有反应条件温和、官能团耐受性好、铜催化剂丰富且可重复使用、易于获得的库存和 O 2 等优点。 作为唯一的氧化剂,为喹啉衍生物的可持续合成提供了另一种途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号