当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Diazirines and 15N2-Diazirines from Ketones, Aldehydes and Derivatives: Development and Mechanistic Insight
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-21 , DOI: 10.1002/adsc.202100679 Quentin Ibert 1 , Madeleine Cauwel 2 , Thomas Glachet 1 , Tony Tite 3 , patricia Le Nahenec-Martel 4 , Jean-Francois Lohier 5 , pierre-yves renard 6 , Xavier Franck 2 , Vincent Reboul 7 , Cyrille Sabot 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-21 , DOI: 10.1002/adsc.202100679 Quentin Ibert 1 , Madeleine Cauwel 2 , Thomas Glachet 1 , Tony Tite 3 , patricia Le Nahenec-Martel 4 , Jean-Francois Lohier 5 , pierre-yves renard 6 , Xavier Franck 2 , Vincent Reboul 7 , Cyrille Sabot 3
Affiliation
|
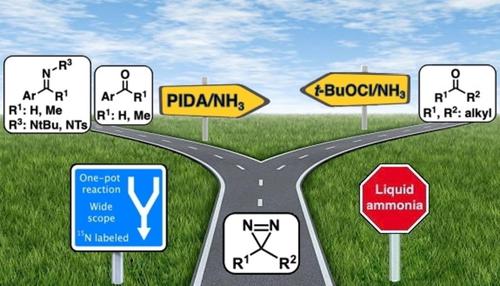
Broad scope one-pot diazirine synthesis strategies have been developed using two different oxidants depending on the nature of the starting material. In all cases, an inexpensive commercial solution of ammonia (NH3) in methanol (MeOH) was employed, avoiding the difficult use of liquid ammonia. With aliphatic ketones, t-butyl hypochlorite (t-BuOCl) was found to be the best oxidant whereas it is preferable to use phenyliodine diacetate (PIDA) with aromatic ketones, aldehydes and imines. The nature of the imine-protecting group is essential and only t-butyl imine allowed the synthesis of 15N2-diazirine with complete 15N incorporation, emphasizing a key trans-imination step in the reaction mechanism. These methods are operationally simple, and tolerant to most functional groups, providing diazirines with yields ranging from 20 to 99%.
中文翻译:

从酮、醛和衍生物中一锅法合成二氮丙啶和 15N2-二氮丙啶:发展和机理洞察
根据起始材料的性质,使用两种不同的氧化剂开发了广泛的一锅二氮嗪合成策略。在所有情况下,均采用廉价的商业氨 (NH 3 ) 甲醇 (MeOH) 溶液,避免了使用液氨的困难。对于脂肪族酮,发现次氯酸叔丁酯 ( t- BuOCl) 是最好的氧化剂,而最好使用带有芳香族酮、醛和亚胺的二乙酸苯碘酯 (PIDA)。亚胺保护基团的性质是必不可少的,只有叔丁基亚胺允许合成15 N 2 -二氮杂环和完整的15N 掺入,强调反应机制中的关键转亚胺化步骤。这些方法操作简单,对大多数官能团都具有耐受性,提供了产率在 20% 到 99% 之间的二氮嗪。
更新日期:2021-09-21
中文翻译:

从酮、醛和衍生物中一锅法合成二氮丙啶和 15N2-二氮丙啶:发展和机理洞察
根据起始材料的性质,使用两种不同的氧化剂开发了广泛的一锅二氮嗪合成策略。在所有情况下,均采用廉价的商业氨 (NH 3 ) 甲醇 (MeOH) 溶液,避免了使用液氨的困难。对于脂肪族酮,发现次氯酸叔丁酯 ( t- BuOCl) 是最好的氧化剂,而最好使用带有芳香族酮、醛和亚胺的二乙酸苯碘酯 (PIDA)。亚胺保护基团的性质是必不可少的,只有叔丁基亚胺允许合成15 N 2 -二氮杂环和完整的15N 掺入,强调反应机制中的关键转亚胺化步骤。这些方法操作简单,对大多数官能团都具有耐受性,提供了产率在 20% 到 99% 之间的二氮嗪。











































 京公网安备 11010802027423号
京公网安备 11010802027423号