Colloid and Interface Science Communications ( IF 4.7 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.colcom.2021.100460 He Xiong 1 , Yang Xiao 1 , Zhiguo Yan 2
|
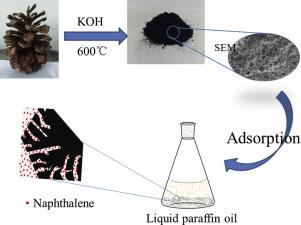
Liquid paraffin oil is harmful to organisms as it contains noxious substances such as aromatic hydrocarbons. Since liquid paraffin oil is difficult to be decomposed in organisms, liquid paraffin oil will eventually reach the human body through the food chain. Besides, the presence of aromatic hydrocarbons in liquid paraffin oil also debases the downstream products. Therefore, it is of great practical value to remove aromatic hydrocarbons in liquid paraffin oil. In this study, pinecone-based activated carbon was prepared to adsorb naphthalene, which is a typical aromatic hydrocarbon in liquid paraffin oil. The prepared adsorbents were characterized by Scanning Electron Microscopy (SEM), N2 adsorption-desorption isotherms, X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). N2 adsorption-desorption isotherms manifested that the specific surface area (SSA) amplified with the KOH/precursor proportion. Although the highest SSA (1191 m2/g) was achieve in the adsorbent with an impregnation rate of 1:4 (KOH/precursor), the sample with an impregnation rate of 1:2 showed the largest adsorption capacity (435 mg/g) of naphthalene. This indicated that the SSA was not directly proportional to the adsorption capacity of naphthalene and the existence of mesoporous was also favorable for adsorption. The adsorption kinetic parameters were fitted with the pseudo-first-order, pseudo-second-order, and intra-particle diffusion models. The adsorption isotherms were fitted by Langmuir and Freundlich models. Finally, the KOH activation mechanism was also discussed.
中文翻译:

活性炭的制备及其对液体石蜡油中萘的吸附
液体石蜡油对生物体有害,因为它含有有毒物质,如芳烃。由于液体石蜡油在生物体内很难被分解,液体石蜡油最终会通过食物链到达人体。此外,液体石蜡油中芳烃的存在也会降低下游产品的价格。因此,去除液体石蜡油中的芳烃具有重要的实用价值。在本研究中,制备了松果基活性炭以吸附萘,萘是液体石蜡油中的典型芳烃。通过扫描电子显微镜(SEM)、N 2吸附-解吸等温线、X射线衍射(XRD)和傅里叶变换红外光谱(FTIR)对制备的吸附剂进行表征。N2吸附-解吸等温线表明比表面积(SSA)随着KOH/前体比例的增加而放大。尽管在浸渍率为 1:4(KOH/前体)的吸附剂中实现了最高的 SSA(1191 m 2 /g),但浸渍率为 1:2 的样品显示出最大的吸附容量(435 mg/g ) 萘。这表明SSA与萘的吸附能力不成正比,介孔的存在也有利于吸附。吸附动力学参数符合拟一级、拟二级和颗粒内扩散模型。吸附等温线由 Langmuir 和 Freundlich 模型拟合。最后,还讨论了 KOH 活化机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号