Cell Chemical Biology ( IF 6.6 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.chembiol.2021.06.009 Daisuke Fujinami 1 , Chantal V Garcia de Gonzalo 1 , Subhanip Biswas 1 , Yue Hao 2 , Huan Wang 1 , Neha Garg 2 , Tiit Lukk 2 , Satish K Nair 2 , Wilfred A van der Donk 3
|
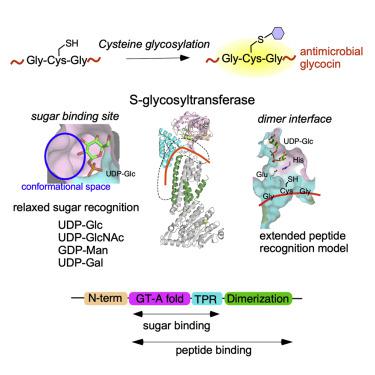
Attachment of sugars to nitrogen and oxygen in peptides is ubiquitous in biology, but glycosylation of sulfur atoms has only been recently described. Here, we characterize two S-glycosyltransferases SunS and ThuS that selectively glycosylate one of five Cys residues in their substrate peptides; substitution of this Cys with Ser results in a strong decrease in glycosylation activity. Crystal structures of SunS and ThuS in complex with UDP-glucose or a derivative reveal an unusual architecture in which a glycosyltransferase type A (GTA) fold is decorated with additional domains to support homodimerization. Dimer formation creates an extended cavity for the substrate peptide, drawing functional analogy with O-glycosyltransferases involved in cell wall biosynthesis. This extended cavity contains a sharp bend that may explain the site selectivity of the glycosylation because the target Cys is in a Gly-rich stretch that can accommodate the bend. These studies establish a molecular framework for understanding the unusual S-glycosyltransferases.
中文翻译:

蛋白质 S-糖基转移酶的结构和机制研究
糖与肽中氮和氧的结合在生物学中普遍存在,但硫原子的糖基化直到最近才被描述。在这里,我们表征了两种S -糖基转移酶 SunS 和 ThuS,它们选择性地糖基化其底物肽中五个 Cys 残基之一;用 Ser 取代此 Cys 会导致糖基化活性的强烈降低。与 UDP-葡萄糖或衍生物复合的 SunS 和 ThuS 的晶体结构揭示了一种不寻常的结构,其中 A 型糖基转移酶 (GTA) 折叠装饰有额外的结构域以支持同二聚化。二聚体的形成为底物肽创造了一个扩展的空腔,与O进行功能类比-参与细胞壁生物合成的糖基转移酶。这个扩展的空腔包含一个尖锐的弯曲,这可以解释糖基化的位点选择性,因为目标 Cys 处于可以适应弯曲的富含甘氨酸的伸展中。这些研究建立了一个分子框架来理解不寻常的S-糖基转移酶。









































 京公网安备 11010802027423号
京公网安备 11010802027423号