当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Products of the Aqueous Phase Oxidation of Triethylamine by OH
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-07-21 , DOI: 10.1021/acsearthspacechem.1c00162 Ye Tao 1 , Tengyu Liu 2 , Xiaoying Yang 2 , Jennifer G. Murphy 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-07-21 , DOI: 10.1021/acsearthspacechem.1c00162 Ye Tao 1 , Tengyu Liu 2 , Xiaoying Yang 2 , Jennifer G. Murphy 2
Affiliation
|
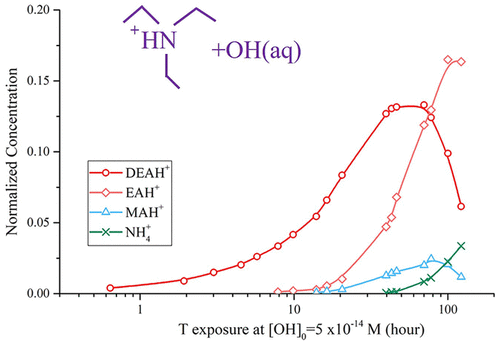
Low-molecular-weight alkyl amines have attracted attention for their roles in new particle formation, ultrafine particle growth, and neutralizing aerosol acidity. The major source of the low-molecular-weight aminium ions in fine particles is generally believed to be the corresponding gaseous precursors emitted to the atmosphere. Relatively little work has investigated whether larger amines can be transformed into smaller amines within the condensed phase itself. In this work, we studied the bulk aqueous phase oxidation kinetics and products of triethylamine/triethylaminium (pKa = 10.75) against OH under different values of pH. Using relative rate kinetics, we find that the oxidation rates against OH for TEA and TEAH+ are (74.3 ± 6.5) × 108 and (1.8 ± 0.2) × 108 M–1 s–1, respectively. In a dilute NaHSO4 solution with a pH of 3.2, the ionic products of TEAH+ oxidation include diethylaminium, ethylaminium, and ammonium ions, which are formed within an atmospherically relevant oxidation time scale. Under common atmospheric conditions, aqueous phase oxidation is calculated to be competitive with gas phase oxidation as a sink for TEA. This indicates that some low-molecular-weight aminium ions can also be the products of the aqueous phase oxidation of larger N-containing molecules.
中文翻译:

OH水相氧化三乙胺的动力学及产物
低分子量烷基胺因其在新颗粒形成、超细颗粒生长和中和气溶胶酸度方面的作用而备受关注。细颗粒中低分子量铵离子的主要来源通常被认为是排放到大气中的相应气态前体。研究较大的胺是否可以在凝聚相本身内转化为较小的胺的工作相对较少。在这项工作中,我们研究了不同 pH 值下三乙胺/三乙胺 (p K a = 10.75) 对 OH的本体水相氧化动力学和产物。使用相对速率动力学,我们发现 TEA 和 TEAH +对 OH 的氧化速率为(74.3 ± 6.5) × 10 8和 (1.8 ± 0.2) × 10 8 M –1 s –1分别。在pH 为 3.2的稀释 NaHSO 4溶液中,TEAH +氧化的离子产物包括二乙胺、乙胺和铵离子,它们是在大气相关的氧化时间尺度内形成的。在常见的大气条件下,水相氧化被计算为与作为 TEA 汇的气相氧化竞争。这表明一些低分子量的胺离子也可能是较大的含氮分子的水相氧化产物。
更新日期:2021-08-19
中文翻译:

OH水相氧化三乙胺的动力学及产物
低分子量烷基胺因其在新颗粒形成、超细颗粒生长和中和气溶胶酸度方面的作用而备受关注。细颗粒中低分子量铵离子的主要来源通常被认为是排放到大气中的相应气态前体。研究较大的胺是否可以在凝聚相本身内转化为较小的胺的工作相对较少。在这项工作中,我们研究了不同 pH 值下三乙胺/三乙胺 (p K a = 10.75) 对 OH的本体水相氧化动力学和产物。使用相对速率动力学,我们发现 TEA 和 TEAH +对 OH 的氧化速率为(74.3 ± 6.5) × 10 8和 (1.8 ± 0.2) × 10 8 M –1 s –1分别。在pH 为 3.2的稀释 NaHSO 4溶液中,TEAH +氧化的离子产物包括二乙胺、乙胺和铵离子,它们是在大气相关的氧化时间尺度内形成的。在常见的大气条件下,水相氧化被计算为与作为 TEA 汇的气相氧化竞争。这表明一些低分子量的胺离子也可能是较大的含氮分子的水相氧化产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号