当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Large, Anionic Liposomes Enable Targeted Intraperitoneal Delivery of a TLR 7/8 Agonist To Repolarize Ovarian Tumors’ Microenvironment
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.bioconjchem.1c00139 Yanan Kang , Linda Flores , Hoi Wa Ngai , Yvonne R. Cornejo , Tom Haber , Marisa McDonald , Dayson Friaça Moreira , Joanna Marie Gonzaga , Wafa Abidi , Yijia Zhang , Mohamed Hammad , Marcin Kortylewski , Karen S. Aboody , Jacob M. Berlin
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.bioconjchem.1c00139 Yanan Kang , Linda Flores , Hoi Wa Ngai , Yvonne R. Cornejo , Tom Haber , Marisa McDonald , Dayson Friaça Moreira , Joanna Marie Gonzaga , Wafa Abidi , Yijia Zhang , Mohamed Hammad , Marcin Kortylewski , Karen S. Aboody , Jacob M. Berlin
|
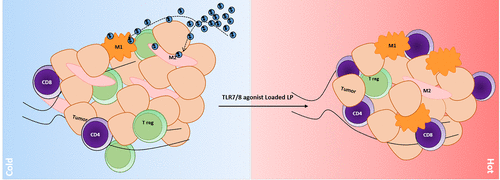
Ovarian cancer is the most lethal gynecological malignancy in the United States. Current standard of treatment includes surgical debulking and chemotherapy, such as cisplatin and paclitaxel. However, the patients’ response rate for chemotherapy in ovarian cancer is not optimal, and they often develop chemoresistance and suffer from side effects. Current clinical trials make extensive use of immune checkpoint blockade (ICB) as a novel cancer immunotherapeutic strategy against ovarian tumors. However, the response rates for ICB antibodies remain limited to 10–20% of treated ovarian cancer patients despite the success of this approach in melanoma, renal, head and neck, and nonsmall cell lung cancers. This lack of efficacy is often attributed to the “cold” immune status of ovarian tumors, as these tumors often have a low number of tumor-infiltrating lymphocytes (TILs) but a high number of suppressive immune cells, including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), or regulatory T cells (Tregs). Repolarizing TAMs could be a promising strategy to reshape the tumor immune microenvironment and promote antitumor activity when combined with ICBs. Toll-like receptor (TLR) 7 and 8 agonists, such as imiquimod and resiquimod, are potent immunostimulatory molecules with potential to repolarize macrophages. However, these small molecules have poor pharmacokinetic profiles and can induce severe side effects when administered systemically. Previously, our group demonstrated that various large, anionic nanomaterials (silica, PLGA, and polystyrene) specifically target TAMs when administered intraperitoneally (IP) to ovarian tumor-bearing mice. In the present study, we demonstrate that large, anionic liposomes administered IP also efficiently localize to TAMs and can be used to target the delivery of resiquimod. Resiquimod delivered in this targeted fashion promoted activation of M1 macrophages and T cell infiltration, while reducing the percentage of Tregs in the tumor microenvironment. Finally, liposome-formulated resiquimod significantly enhanced the efficacy of PD1 blockade against syngeneic ovarian tumors. We anticipate that further optimization of our liposomal delivery strategy can generate a clinically relevant strategy for more effective and safer immunotherapy for ovarian cancer patients.
中文翻译:

大的阴离子脂质体能够靶向腹膜内递送 TLR 7/8 激动剂以重新极化卵巢肿瘤的微环境
卵巢癌是美国最致命的妇科恶性肿瘤。目前的治疗标准包括手术减瘤和化疗,如顺铂和紫杉醇。然而,卵巢癌患者对化疗的反应率并不理想,往往会产生耐药性并出现副作用。目前的临床试验广泛使用免疫检查点阻断 (ICB) 作为一种针对卵巢肿瘤的新型癌症免疫治疗策略。然而,尽管这种方法在黑色素瘤、肾癌、头颈癌和非小细胞肺癌中取得了成功,但 ICB 抗体的反应率仍然仅限于 10-20% 的接受治疗的卵巢癌患者。这种缺乏疗效通常归因于卵巢肿瘤的“冷”免疫状态,因为这些肿瘤通常具有少量的肿瘤浸润淋巴细胞 (TIL) 但具有大量抑制性免疫细胞,包括肿瘤相关巨噬细胞 (TAM)、髓源性抑制细胞 (MDSC) 或调节性 T 细胞 (Treg) . 复极化 TAMs 与 ICBs 结合可能是重塑肿瘤免疫微环境和促进抗肿瘤活性的一种有前景的策略。Toll 样受体 (TLR) 7 和 8 激动剂,如咪喹莫特和瑞喹莫特,是具有重新极化巨噬细胞潜力的有效免疫刺激分子。然而,这些小分子具有较差的药代动力学特征,并且在全身给药时会引起严重的副作用。此前,我们的团队证明了各种大型阴离子纳米材料(二氧化硅、PLGA、和聚苯乙烯)在腹膜内 (IP) 给予卵巢肿瘤小鼠时特异性靶向 TAM。在本研究中,我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。
更新日期:2021-08-19
中文翻译:

大的阴离子脂质体能够靶向腹膜内递送 TLR 7/8 激动剂以重新极化卵巢肿瘤的微环境
卵巢癌是美国最致命的妇科恶性肿瘤。目前的治疗标准包括手术减瘤和化疗,如顺铂和紫杉醇。然而,卵巢癌患者对化疗的反应率并不理想,往往会产生耐药性并出现副作用。目前的临床试验广泛使用免疫检查点阻断 (ICB) 作为一种针对卵巢肿瘤的新型癌症免疫治疗策略。然而,尽管这种方法在黑色素瘤、肾癌、头颈癌和非小细胞肺癌中取得了成功,但 ICB 抗体的反应率仍然仅限于 10-20% 的接受治疗的卵巢癌患者。这种缺乏疗效通常归因于卵巢肿瘤的“冷”免疫状态,因为这些肿瘤通常具有少量的肿瘤浸润淋巴细胞 (TIL) 但具有大量抑制性免疫细胞,包括肿瘤相关巨噬细胞 (TAM)、髓源性抑制细胞 (MDSC) 或调节性 T 细胞 (Treg) . 复极化 TAMs 与 ICBs 结合可能是重塑肿瘤免疫微环境和促进抗肿瘤活性的一种有前景的策略。Toll 样受体 (TLR) 7 和 8 激动剂,如咪喹莫特和瑞喹莫特,是具有重新极化巨噬细胞潜力的有效免疫刺激分子。然而,这些小分子具有较差的药代动力学特征,并且在全身给药时会引起严重的副作用。此前,我们的团队证明了各种大型阴离子纳米材料(二氧化硅、PLGA、和聚苯乙烯)在腹膜内 (IP) 给予卵巢肿瘤小鼠时特异性靶向 TAM。在本研究中,我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。我们证明 IP 给药的大型阴离子脂质体也有效地定位于 TAM,可用于靶向传递瑞喹莫特。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。以这种靶向方式递送的 Resiquimod 促进了 M1 巨噬细胞的激活和 T 细胞浸润,同时降低了肿瘤微环境中 Treg 的百分比。最后,脂质体配方的瑞喹莫特显着增强了 PD1 阻断对同系卵巢肿瘤的疗效。我们预计,进一步优化我们的脂质体递送策略可以为卵巢癌患者提供更有效和更安全的免疫治疗的临床相关策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号