Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Formal Synthesis of Trichodermamides B and C
Synlett ( IF 1.7 ) Pub Date : 2021-07-21 , DOI: 10.1055/s-0040-1720383 Atsushi Kimishima , Masayoshi Arai , Hayato Saito , Violeta Petrova
中文翻译:

木霉酰胺 B 和 C 的不对称形式合成
更新日期:2021-07-22
Synlett ( IF 1.7 ) Pub Date : 2021-07-21 , DOI: 10.1055/s-0040-1720383 Atsushi Kimishima , Masayoshi Arai , Hayato Saito , Violeta Petrova
|
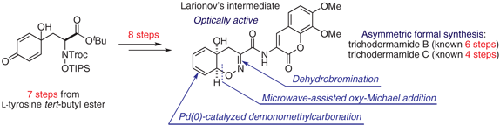
An asymmetric formal synthesis of trichodermamides B and C was achieved in 15 steps based on a tyrosine ester chiral-pool approach. Key features of this synthesis include stereoselective construction of a cis-1,2-oxazadecaline core by an acid-mediated tandem deprotection–intramolecular oxy-Michael reaction, oxime ether formation via an N-bromination–elimination sequence, and diene construction by a palladium-catalyzed demonomethylcarbonation.
中文翻译:

木霉酰胺 B 和 C 的不对称形式合成
基于酪氨酸酯手性池方法,木霉酰胺 B 和 C 的不对称形式合成通过 15 个步骤实现。该合成的关键特征包括通过酸介导的串联脱保护-分子内氧-迈克尔反应立体选择性构建顺式-1,2-氧氮杂萘核心,通过N-溴化-消除序列形成肟醚,以及通过钯构建二烯-催化的去甲甲基碳酸化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号