Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.jinorgbio.2021.111554 Matteo Masetti 1 , Martina Bertazzo 2 , Maurizio Recanatini 1 , Stefano Ciurli 3 , Francesco Musiani 3
|
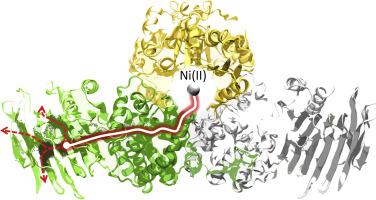
The survival of several pathogenic bacteria, such as Helicobacter pylori (Hp), relies on the activity of the nickel-dependent enzyme urease. Nickel insertion into urease is mediated by a multimeric chaperone complex (HpUreDFG) that is responsible for the transport of Ni(II) from a conserved metal binding motif located in the UreG dimer (CPH motif) to the catalytic site of the enzyme. The X-ray structure of HpUreDFG revealed the presence of water-filled tunnels that were proposed as a route for Ni(II) translocation. Here, we probe the transport of Ni(II) through the internal tunnels of HpUreDFG, from the CPH motif to the external surface of the complex, using microsecond-long enhanced molecular dynamics simulations. The results suggest a “bucket-brigade” mechanism whereby Ni(II) can be transported through a series of stations found along these internal pathways.
中文翻译:

探索 Ni (II) 离子通过幽门螺杆菌 UreDFG 多聚蛋白复合物内部通道的运输
几种致病细菌,如幽门螺杆菌( Hp ) 的存活依赖于镍依赖酶脲酶的活性。镍插入脲酶由多聚体伴侣复合物 ( Hp UreDFG) 介导,该复合物负责将 Ni(II) 从位于 UreG 二聚体(CPH 基序)中的保守金属结合基序转运到酶的催化位点。Hp UreDFG的 X 射线结构揭示了充满水的隧道的存在,这些隧道被提议作为 Ni(II) 易位的途径。在这里,我们探讨了 Ni(II) 通过Hp内部隧道的传输UreDFG,从 CPH 基序到复合物的外表面,使用微秒长的增强分子动力学模拟。结果表明,Ni(II) 可以通过沿这些内部路径发现的一系列站点运输“桶式”机制。










































 京公网安备 11010802027423号
京公网安备 11010802027423号