Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.jinorgbio.2021.111541 Melanie Paul 1 , Melissa Teubner 2 , Benjamin Grimm-Lebsanft 3 , Sören Buchenau 3 , Alexander Hoffmann 1 , Michael Rübhausen 3 , Sonja Herres-Pawlis 1
|
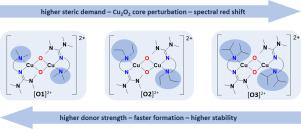
The tyrosinase-like activity of hybrid guanidine-stabilized bis(μ-oxido) dicopper(III) complexes [Cu2(μ-O)2(L)2](X)2 (L = 2-{2-((Diethylamino)methyl)phenyl}-1,1,3,3-tetramethylguanidine (TMGbenzNEt2, L2) and 2-{2-((Di-isopropylamino)methyl)phenyl}-1,1,3,3-tetramethylguanidine (TMGbenzNiPr2, L3); X = PF6−, BF4−, CF3SO3−) is described. New aromatic hybrid guanidine amine ligands were developed with varying amine donor function. Their copper(I) complexes were analyzed towards their ability to activate dioxygen in the presence of different weakly coordinating anions. The resulting bis(μ-oxido) species were characterized at low temperatures by UV/Vis and resonance Raman spectroscopy, cryo-ESI mass spectrometry and density functional theory calculations. Small structural changes in the ligand sphere were found to influence the characteristic ligand-to-metal charge transfer (LMCT) features of the bis(μ-oxido) species, correlating a redshift in the UV/Vis spectrum with weaker N-donor function of the ligand. DFT calculations elucidated the influence of the steric and electronic properties of the bis(μ-oxido) species leading to a higher twist of the Cu2O2 plane against the CuN2 plane and a stretching of the Cu2O2 core. Despite their moderate stability at −100 °C, the bis(μ-oxido) complexes exhibited a remarkable activity in catalytic oxygenation reactions of polycyclic aromatic alcohols. Further the selectivity of the catalyst in the hydroxylation reactions of challenging phenolic substrates is not changed despite an increasing shield of the reactive bis(μ-oxido) core. The generated quinones were found to form exclusively bent phenazines, providing a promising strategy to access tailored phenazine derivatives.
中文翻译:

胺供体对杂化胍稳定的双(μ-氧化)二铜(III)配合物及其对多环芳香醇类酪氨酸酶氧化活性的影响
杂合胍稳定的双(μ-氧化)二铜(III)复合物[Cu 2 (μ-O) 2 (L) 2 ](X) 2 (L = 2-{2-((二乙氨基))的酪氨酸酶样活性)甲基)苯基}-1,1,3,3-四甲基胍(TMGbenzNEt 2 , L2 )和2-{2-((二异丙基氨基)甲基)苯基}-1,1,3,3-四甲基胍(TMGbenzN我Pr 2 , L3 ); X = PF 6 - , BF 4 - , CF 3 SO 3 -) 进行了描述。开发了具有不同胺供体功能的新型芳香杂化胍胺配体。分析了它们的铜 (I) 配合物在不同弱配位阴离子存在下激活分子氧的能力。通过紫外/可见和共振拉曼光谱、低温-ESI 质谱和密度泛函理论计算,在低温下对所得双 (μ-氧化) 物质进行了表征。发现配体球体中的微小结构变化会影响双(μ-氧化物)物种的特征性配体到金属电荷转移(LMCT)特征,将UV / Vis光谱中的红移与较弱的N-供体功能相关联配体。DFT计算阐明了双(μ-氧化物)物种的空间和电子特性的影响,导致Cu的更高扭曲2 O 2平面对着CuN 2平面和Cu 2 O 2核心的拉伸。尽管在-100°C下具有中等稳定性,但双(μ-氧化)配合物在多环芳族醇的催化氧化反应中表现出显着的活性。此外,尽管活性双(μ-氧化)核的屏蔽增加,但催化剂在具有挑战性的酚醛底物的羟基化反应中的选择性没有改变。发现生成的醌仅形成弯曲的吩嗪,为获得定制的吩嗪衍生物提供了一种有前景的策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号