Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.jiec.2021.07.025 Linping Yu 1 , Long Chen 1 , Qizhi Chen 2 , Luli Feng 3 , Ziyi Xu 1 , Bo Nan 4 , Xiyue Kang 3 , Yuehui He 3
|
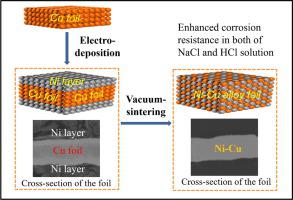
Cost-effective ultrathin alloy foils (<20 μm) are highly expected with the development of electronic industry and micro-system technology. In this paper, electrodeposition combined with vacuum sintering is used to fabricate a Ni-Cu alloy foil with thickness of 12.0 (±0.2) μm. For the ultrathin Ni-Cu alloy foil, a densified structure without pores can be achieved by prolonging sintering duration at 900 ℃ for 3 h. Under the current density of 10 mA cm−2, 700 s is the optimal electrodeposition time to obtain the highest tensile strength (187 MPa) with the Ni content of 41.5 wt.% in the alloy foil. Compared with Cu foil, Ni-Cu alloy foil shows superior corrosion resistance in 3.5 wt.% NaCl solution and also HCl solutions (0.5 mol/L, 1.0 mol/L, 2.0 mol/L), respectively. The uniform composition and defect-free surface, excellent tensile strength and corrosion resistance together exhibits the great application potential of the obtained Ni-Cu alloy foil, which may provide an inspiration for future development of integrated electronic or medical devices.
中文翻译:

超薄镍铜合金箔的可控制备及电化学腐蚀行为
随着电子工业和微系统技术的发展,人们对具有成本效益的超薄合金箔(<20 μm)寄予厚望。本文采用电沉积结合真空烧结的方法制备了厚度为 12.0 (±0.2) μm 的 Ni-Cu 合金箔。对于超薄Ni-Cu合金箔,通过延长900 ℃ 3 h的烧结时间,可以获得无气孔的致密结构。10 mA cm -2电流密度下, 700 s 是获得最高拉伸强度 (187 MPa) 的最佳电沉积时间,合金箔中的 Ni 含量为 41.5 wt.%。与 Cu 箔相比,Ni-Cu 合金箔分别在 3.5 wt.% NaCl 溶液和 HCl 溶液(0.5 mol/L、1.0 mol/L、2.0 mol/L)中表现出优异的耐腐蚀性。均匀的成分和无缺陷的表面、优异的抗拉强度和耐腐蚀性共同展示了所获得的Ni-Cu合金箔的巨大应用潜力,这可能为集成电子或医疗器械的未来发展提供启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号