Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.jhazmat.2021.126650 Xubo Huang 1 , Min Chen 1 , Yaru Wang 1 , Chen Chen 1 , Yiming Xu 1
|
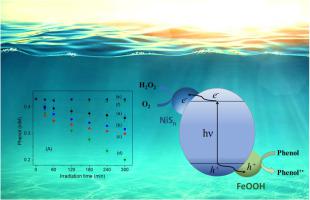
Monoclinic BiVO4 (BiV) has been widely used as a photoanode for water oxidation, but rarely as a photocatalyst for organic oxidation due to slow reaction of O2. In this work, BiV has been modified with poorly crystallized sFe and sNi, where sFe is FeOOH, and sNi is a mixture of Ni(OH)2 and polysulfide. Under light, sFe/BiV and sNi/BiV in aqueous solution were more active than BiV, respectively, not only for phenol oxidation but also for O2 reduction. Importantly, the rate of phenol oxidation obtained for sFe/sNi/BiV was larger than the sum of the rates measured for sFe/BiV and sNi/BiV, by a factor of approximately 1.5. Moreover, on a film electrode, O2 reduction had a current of sFe/sNi/BiV > sNi/BiV > sFe/BiV > BiV, while water (photo)oxidation had a current of sFe/sNi/BiV > sNi/BiV > sFe/BiV > BiV. A possible mechanism is proposed, involving formation of a reduced sulfur species for O2 reduction and an oxidized iron species for phenol oxidation. In sFe/sNi/BiV, there is a mutual promotion between the sNi-mediated electron transfer and the sFe-mediated hole transfer. This results in a further improved efficiency of charge separation for O2 reduction and phenol oxidation.
中文翻译:

无定形NiSn和FeOOH作为可见光下BiVO4上氧还原和苯酚(水)氧化的双功能助催化剂
单斜 BiVO 4 (BiV) 已被广泛用作水氧化的光阳极,但由于 O 2反应缓慢,很少用作有机氧化的光催化剂。在这项工作中,BiV 用结晶较差的 sFe 和 sNi 进行了改性,其中 sFe 是 FeOOH,而 sNi 是 Ni(OH) 2和多硫化物的混合物。在光照下,水溶液中的 sFe/BiV 和 sNi/BiV 分别比 BiV 更活跃,不仅对苯酚氧化,而且对 O 2还原也如此。重要的是,sFe/sNi/BiV 获得的苯酚氧化速率比 sFe/BiV 和 sNi/BiV 测得的速率之和大约 1.5 倍。此外,在薄膜电极上,O 2还原的电流为 sFe/sNi/BiV > sNi/BiV > sFe/BiV > BiV,而水(光)氧化的电流为 sFe/sNi/BiV > sNi/BiV > sFe/BiV > BiV。提出了一种可能的机制,包括形成用于 O 2还原的还原硫物质和用于苯酚氧化的氧化铁物质。在 sFe/sNi/BiV 中,sNi 介导的电子转移和 sFe 介导的空穴转移相互促进。这导致O 2还原和苯酚氧化的电荷分离效率进一步提高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号