当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of 5-Fluorocytosine in Different Pure and Binary Mixed Solvents: Measurement, Model Correlation, Solvent Effect, and Preferential Solvation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.jced.1c00206 Yang Cong 1 , Cunbin Du 1 , Meng Wang 1 , Zhouyu Jiang 1 , Mingliang Wang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.jced.1c00206 Yang Cong 1 , Cunbin Du 1 , Meng Wang 1 , Zhouyu Jiang 1 , Mingliang Wang 1
Affiliation
|
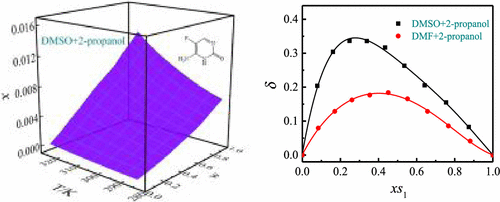
In this work, the phase equilibrium solubility of 5-fluorocytosine (5-FC) in dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), 1-butanol, acetonitrile, 1-propanol, 1,4-dioxane, acetone, ethyl acetate, 2-propanol, 2-butanone, and mixture systems (DMSO + 2-propanol and DMF + 2-propanol) at a series of temperatures (283.15–323.15 K) is determined, and model correlation, solvent effect, and preferential solvation analysis are researched. The solubility of 5-FC is related to temperature, which decreases with decreasing temperature. For monosolvent systems, the mole solubility order is DMSO > DMF > 1-butanol > 1-propanol > 2-propanol > acetone > 2-butanone > 1,4-dioxane > acetonitrile > ethyl acetate. The solvent effect has been studied in monosolvents, hydrogen-bonding acceptor (HBA) interactions and nonspecific dipolarity/polarizability interactions favor dissolution, and solvent self-cohesiveness is not beneficial to dissolution. For two mixture systems, the mole solubility of 5-FC in DMSO + 2-propanol solvent systems is higher than that in DMF + 2-propanol solvent systems. A dimensionless parameter (δ) is defined to observe the deviation from ideality of mixed solvents whose values are all greater than zero. The equilibrium constant of the solvent exchange process (Kps) can be used to evaluate the preferential solvation of 5-FC. DMSO or DMF dissolves 5-FC in the local region around 5-FC preferentially. The equilibrium solubility data is correlated by four correlation models (modified Apelblat, λh, CNIBS/R-K, and Jouyban–Acree models). The greatest relative average deviation (RAD) and root-mean-square deviation (RMSD) values are 2.01% and 11.87 × 10–5 in pure solvent systems and 2.20% and 5.99 × 10–5 in mixture systems, respectively. These four models show excellent correlation with the experimental solubility data.
中文翻译:

5-氟胞嘧啶在不同纯溶剂和二元混合溶剂中的溶解度:测量、模型关联、溶剂效应和优先溶剂化
在这项工作中,5-氟胞嘧啶 (5-FC) 在二甲基亚砜 (DMSO) 中的相平衡溶解度,N,N-二甲基甲酰胺 (DMF)、1-丁醇、乙腈、1-丙醇、1,4-二恶烷、丙酮、乙酸乙酯、2-丙醇、2-丁酮和混合系统(DMSO + 2-丙醇和 DMF + 2-丙醇)确定了一系列温度 (283.15–323.15 K) 下的 ,并研究了模型相关性、溶剂效应和优先溶剂化分析。5-FC 的溶解度与温度有关,随着温度的降低而降低。对于单溶剂系统,摩尔溶解度顺序为 DMSO > DMF > 1-丁醇 > 1-丙醇 > 2-丙醇 > 丙酮 > 2-丁酮 > 1,4-二恶烷 > 乙腈 > 乙酸乙酯。已经在单溶剂中研究了溶剂效应,氢键受体 (HBA) 相互作用和非特异性偶极/极化相互作用有利于溶解,溶剂自粘性不利于溶解。对于两种混合体系,5-FC在DMSO+2-丙醇溶剂体系中的摩尔溶解度高于在DMF+2-丙醇溶剂体系中的摩尔溶解度。定义无量纲参数 (δ) 以观察其值均大于零的混合溶剂与理想情况的偏差。溶剂交换过程的平衡常数(K ps ) 可用于评估 5-FC 的优先溶剂化。DMSO 或 DMF 优先溶解 5-FC 周围的局部区域中的 5-FC。平衡溶解度数据通过四个相关模型(修正的 Apelblat、λ h、CNIBS/RK 和 Jouyban-Acree 模型)相关联。最大的相对平均偏差 (RAD) 和均方根偏差 (RMSD) 值在纯溶剂系统中分别为 2.01% 和 11.87 × 10 –5,在混合系统中分别为2.20% 和 5.99 × 10 –5。这四个模型与实验溶解度数据显示出极好的相关性。
更新日期:2021-08-12
中文翻译:

5-氟胞嘧啶在不同纯溶剂和二元混合溶剂中的溶解度:测量、模型关联、溶剂效应和优先溶剂化
在这项工作中,5-氟胞嘧啶 (5-FC) 在二甲基亚砜 (DMSO) 中的相平衡溶解度,N,N-二甲基甲酰胺 (DMF)、1-丁醇、乙腈、1-丙醇、1,4-二恶烷、丙酮、乙酸乙酯、2-丙醇、2-丁酮和混合系统(DMSO + 2-丙醇和 DMF + 2-丙醇)确定了一系列温度 (283.15–323.15 K) 下的 ,并研究了模型相关性、溶剂效应和优先溶剂化分析。5-FC 的溶解度与温度有关,随着温度的降低而降低。对于单溶剂系统,摩尔溶解度顺序为 DMSO > DMF > 1-丁醇 > 1-丙醇 > 2-丙醇 > 丙酮 > 2-丁酮 > 1,4-二恶烷 > 乙腈 > 乙酸乙酯。已经在单溶剂中研究了溶剂效应,氢键受体 (HBA) 相互作用和非特异性偶极/极化相互作用有利于溶解,溶剂自粘性不利于溶解。对于两种混合体系,5-FC在DMSO+2-丙醇溶剂体系中的摩尔溶解度高于在DMF+2-丙醇溶剂体系中的摩尔溶解度。定义无量纲参数 (δ) 以观察其值均大于零的混合溶剂与理想情况的偏差。溶剂交换过程的平衡常数(K ps ) 可用于评估 5-FC 的优先溶剂化。DMSO 或 DMF 优先溶解 5-FC 周围的局部区域中的 5-FC。平衡溶解度数据通过四个相关模型(修正的 Apelblat、λ h、CNIBS/RK 和 Jouyban-Acree 模型)相关联。最大的相对平均偏差 (RAD) 和均方根偏差 (RMSD) 值在纯溶剂系统中分别为 2.01% 和 11.87 × 10 –5,在混合系统中分别为2.20% 和 5.99 × 10 –5。这四个模型与实验溶解度数据显示出极好的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号