Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.seppur.2021.119329 Jing Fu 1, 2 , Fubing Yao 1, 2 , Ting Xie 3 , Yu Zhong 4 , Ziletao Tao 4 , Shengjie Chen 1, 2 , Li He 1, 2 , Zhoujie Pi 1, 2 , Kunjie Hou 1, 2 , Dongbo Wang 1, 2 , Xiaoming Li 1, 2 , Qi Yang 1, 2
|
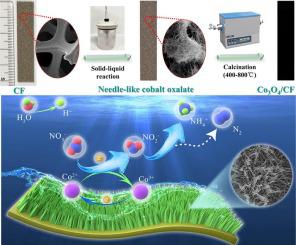
Although Co3O4-based non-noble metal electrodes have caused wide concern in electrochemical denitrification, the electrocatalytic activity and stability of these materials are still unsatisfied. In this work, a self-supported electrode (Co3O4/CF) was first fabricated via in-situ growth of needle-like Co3O4 on the cobalt foam (CF) and then used as cathode for electrochemical denitrification. The physicochemical and electrochemical characters of Co3O4/CF could be regulated by calcination temperature. Owing to the needle-like structure and the internal contact between Co3O4 and CF, the as-prepared Co3O4/CF-600 electrode exhibited excellent electrochemical performances for NO3– removal. The influences of current density, initial NO3– concentration, solution pH, and additive Cl- concentration on electrochemical NO3– reduction were considered. A high NO3– removal efficiency (72.9%) and N2 selectivity (96.2%) were achieved by Co3O4/CF-600 electrode under the optimum conditions: current density 5 mA/cm2, Cl- concentration 1500 mg/L and initial NO3– concentration 50 mg N/L. The cyclic voltammetry (CV) and electrochemical impedance spectra (EIS) confirmed that electrochemical denitrification was mainly realized via Co2+–Co3+–Co2+ redox process instead of H*-mediated indirect process. Moreover, the Co3O4/CF electrode material could keep its electrochemical properties even after 10 cycles.
中文翻译:

在钴泡沫上原位生长针状 Co3O4 作为电化学还原硝酸盐的自支撑阴极
尽管Co 3 O 4基非贵金属电极在电化学反硝化方面引起了广泛关注,但这些材料的电催化活性和稳定性仍不令人满意。在这项工作中,首先通过在钴泡沫 (CF) 上原位生长针状 Co 3 O 4制备自支撑电极 (Co 3 O 4 / CF),然后将其用作电化学反硝化的阴极。Co 3 O 4 /CF的物理化学和电化学特性可以通过煅烧温度来调节。由于针状结构和 Co 3 O之间的内部接触4和CF,所制备的钴3 ö 4 / CF-600电极显示出优异的电化学性能为NO 3 -的去除。考虑了电流密度、初始NO 3 -浓度、溶液pH 和添加剂Cl -浓度对电化学NO 3 -还原的影响。在最佳条件下,Co 3 O 4 /CF-600 电极实现了高 NO 3 –去除效率(72.9%)和 N 2选择性(96.2%):电流密度 5 mA/cm 2,Cl -浓度 1500 mg/L 和初始 NO 3 –浓度 50 mg N/L。循环伏安法(CV)和电化学阻抗谱(EIS)证实电化学反硝化主要通过Co 2+ –Co 3+ –Co 2+氧化还原过程而不是H*介导的间接过程实现。此外,Co 3 O 4 /CF 电极材料即使在 10 次循环后仍能保持其电化学性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号