Energy Conversion and Management ( IF 9.9 ) Pub Date : 2021-07-20 , DOI: 10.1016/j.enconman.2021.114523 Zhi Ying , Zhen Geng , Xiaoyuan Zheng , Binlin Dou , Guomin Cui
|
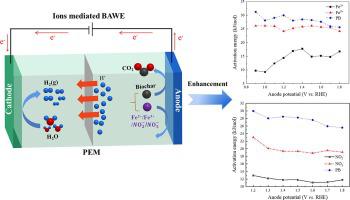
The biochar-assisted water electrolysis (BAWE) is a promising method for clean biomass utilization and hydrogen production. But it is limited by the slow anode biochar oxidation reaction (BOR). To enhance the BOR, the mediator of Fe2+/Fe3+ or / was added to the pyrolytic biochar (PB) and tested using electrochemical methods. Both Fe2+ and showed stronger oxidation activity than Fe3+ and , and thereby presented more significant enhanced role on BOR, including the reduction in onset potential and the enlargement of current density. Fe2+/Fe3+ existence resulted in the five-step mechanism such as adsorption, redox reaction in solution, oxidation at electrode surface, and passive films formation on the biochar surface. 0.5 mol/L or less of Fe2+/Fe3+ was suggestable, while the lower acid concentration was necessary to avoid side reaction with existence. The Fe2+/Fe3+ acted as catalysts due to the almost unchanged total iron ions during continuous BAWE process, whereas a high consumption of occurred. The cathode hydrogen production was less influenced by mediator and showed comparable with the theoretical value of Faraday’s law. In addition, the activation energy of BOR was significantly reduced with Fe2+ and mediation. The present work provides a favorable strategy for improving BOR and energy-saving production of hydrogen via BAWE process.
中文翻译:

用Fe 2+ /Fe 3+或Fe 3+介质增强生物炭氧化反应/ 通过生物炭辅助水电解高效制氢
生物炭辅助水电解(BAWE)是清洁生物质利用和制氢的一种很有前景的方法。但它受到缓慢阳极生物炭氧化反应(BOR)的限制。为了提高 BOR,Fe 2+ /Fe 3+或/被添加到热解生物炭 (PB) 中并使用电化学方法进行测试。Fe 2+和表现出比 Fe 3+更强的氧化活性和,从而对 BOR 表现出更显着的增强作用,包括起始电位的降低和电流密度的增大。Fe 2+ /Fe 3+ 的存在导致了吸附、溶液中氧化还原反应、电极表面氧化和生物炭表面钝化膜形成等五步机制。建议使用 0.5 mol/L 或更少的 Fe 2+ /Fe 3+,而较低的酸浓度是必要的,以避免与存在。Fe 2+ /Fe 3+由于在连续 BAWE 过程中几乎没有变化的总铁离子而起到催化剂的作用,而高消耗的发生。阴极产氢受介质影响较小,与法拉第定律的理论值相当。此外,Fe 2+和BOR 的活化能显着降低。调解。目前的工作为通过BAWE工艺提高BOR和节能制氢提供了有利的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号