Minerals Engineering ( IF 4.8 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.mineng.2021.107067 Jianhua Chen 1
|
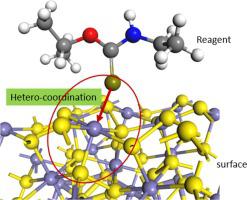
The selective interaction between reagents and mineral surfaces is the core basis of mineral flotation separation. This paper creatively proposes a perspective from coordination chemistry to clarify the interaction mechanism of reagents with mineral surfaces systematically and profoundly. The metal ion in mineral surface is far different from the free ion. The former is in a semi-constrained state, causing the properties of surface metal ions to be greatly affected by surface structures and properties of surrounding atoms. Based on coordination chemistry, the π-backbonding model is advanced for the interaction between sulfhydryl collectors and sulphide minerals. It is of interest that with more π electron pairs, the surface metal ion is more likely to interact with sulfhydryl collectors containing unoccupied π orbitals; with greater polarizability, the metal ion is more prone to covalent interactions with sulfhydryl collectors. In addition, the unoccupied orbitals play a crucial role in selectivity of depressants. For example, the depressants NaCN and Ca(OH)+ containing unoccupied π orbitals can strongly depress pyrite holding π electron pairs, but can hardly depress galena possessing no π electron pair. Furthermore, the crystal field stabilization energy resulting from the interaction between reagents and surface metal ions can influence the stability of reagent adsorption, and can adequately explain the order of flotation critical pH for sulphide minerals. The coordination theory sheds new light on the interaction mechanism between flotation reagents and mineral surfaces.
中文翻译:

浮选剂与矿物表面金属离子的相互作用:从配位化学看
试剂与矿物表面之间的选择性相互作用是矿物浮选分离的核心基础。本文创造性地从配位化学的角度,系统而深刻地阐明了试剂与矿物表面的相互作用机理。矿物表面的金属离子与游离离子相差甚远。前者处于半约束状态,导致表面金属离子的性质受表面结构和周围原子性质的影响很大。基于配位化学,π-backbonding 模型是先进的巯基捕收剂和硫化矿物之间的相互作用。有趣的是,具有更多的 π 电子对,表面金属离子更有可能与包含未占据的 π 轨道的巯基收集器相互作用;具有更大的极化率,金属离子更容易与巯基捕收剂发生共价相互作用。此外,未占据的轨道在抑制剂的选择性中起着至关重要的作用。例如,抑制剂 NaCN 和 Ca(OH)+包含未占据的 π 轨道可以强烈抑制具有 π 电子对的黄铁矿,但几乎不能抑制不具有 π 电子对的方铅矿。此外,试剂与表面金属离子相互作用产生的晶场稳定能会影响试剂吸附的稳定性,可以充分解释硫化物矿物浮选临界pH值的顺序。配位理论揭示了浮选药剂与矿物表面之间的相互作用机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号