当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Antifungal Activity Evaluation of Phloeodictine Analogues
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-07-20 , DOI: 10.1021/acs.jnatprod.1c00116 Ranga Rao Ravu 1 , Melissa R Jacob 1 , Shabana I Khan 1, 2 , Mei Wang 3 , Liang Cao 1 , Ameeta K Agarwal 1, 2 , Alice M Clark 1, 2 , Xing-Cong Li 1, 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-07-20 , DOI: 10.1021/acs.jnatprod.1c00116 Ranga Rao Ravu 1 , Melissa R Jacob 1 , Shabana I Khan 1, 2 , Mei Wang 3 , Liang Cao 1 , Ameeta K Agarwal 1, 2 , Alice M Clark 1, 2 , Xing-Cong Li 1, 2
Affiliation
|
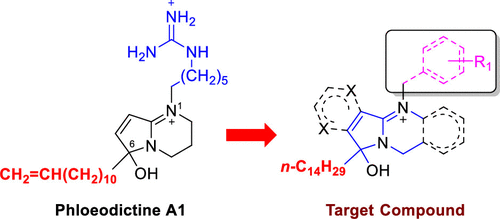
The phloeodictine-based 6-hydroxy-2,3,4,6-tetrahydropyrrolo[1,2-a]pyrimidinium structural moiety with an n-tetradecyl side chain at C-6 has been demonstrated to be a new antifungal template. Thirty-four new synthetic analogues with modifications of the bicyclic tetrahydropyrrolopyrimidinium skeleton and the N-1 side chain have been prepared and evaluated for in vitro antifungal activities against the clinically important fungal pathogens including Cryptococcus neoformans ATCC 90113, Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, and Aspergillus fumigatus ATCC 90906. Nineteen compounds (5, 21–31, 34–38, 44, and 48) showed antifungal activities against the aforementioned five fungal pathogens with minimum inhibitory concentrations (MICs) in the range 0.88–10 μM, and all were fungicidal with minimum fungicidal concentrations (MFCs) similar to the respective MIC values. Compounds 24, 36, and 48 were especially active against C. neoformans ATCC 90113 with MIC/MFC values of 1.0/1.0, 1.6/1.6, and 1.3/2.0 μM but exhibited low cytotoxicity with an IC50 > 40 μM against the mammalian Vero cells. The structure and antifungal activity relationship indicates that synthetic modifications of the phloeodictines can afford analogues with potent antifungal activity and reduced cytotoxicity, necessitating further preclinical studies of this new class of antifungal compounds.
中文翻译:

腐皮碱类似物的合成及抗真菌活性评价
已证明基于 6-羟基-2,3,4,6-四氢吡咯并[1,2- a ]嘧啶鎓结构部分在 C-6 处具有正十四烷基侧链,是一种新的抗真菌模板。三十四个与双环tetrahydropyrrolopyrimidinium骨架和N-1侧链的修饰新合成类似物已被制备并在体外抗真菌活性评价对临床上重要的真菌性病原体,包括新型隐球菌ATCC 90113,白色念珠菌ATCC 90028,光滑念珠菌ATCC 90030、克柔念珠菌ATCC 6258 和烟曲霉ATCC 90906。十九种化合物 ( 5 ,21 – 31、34 – 38、44和48 ) 对上述五种真菌病原体显示出抗真菌活性,最小抑制浓度 (MIC) 范围为 0.88–10 μM,并且均具有杀真菌活性,最小杀真菌浓度 (MFC) 类似于相应的 MIC 值。化合物24,36,和48是特别有效对抗隐球菌ATCC 90113具有1.0 / 1.0的MIC / MFC值,1.6 / 1.6和1.3 / 2.0μM但显示出低细胞毒性,其IC 50> 40 μM 对抗哺乳动物 Vero 细胞。结构和抗真菌活性的关系表明,韧皮素的合成修饰可以提供具有有效抗真菌活性和降低细胞毒性的类似物,需要对这类新的抗真菌化合物进行进一步的临床前研究。
更新日期:2021-08-27
中文翻译:

腐皮碱类似物的合成及抗真菌活性评价
已证明基于 6-羟基-2,3,4,6-四氢吡咯并[1,2- a ]嘧啶鎓结构部分在 C-6 处具有正十四烷基侧链,是一种新的抗真菌模板。三十四个与双环tetrahydropyrrolopyrimidinium骨架和N-1侧链的修饰新合成类似物已被制备并在体外抗真菌活性评价对临床上重要的真菌性病原体,包括新型隐球菌ATCC 90113,白色念珠菌ATCC 90028,光滑念珠菌ATCC 90030、克柔念珠菌ATCC 6258 和烟曲霉ATCC 90906。十九种化合物 ( 5 ,21 – 31、34 – 38、44和48 ) 对上述五种真菌病原体显示出抗真菌活性,最小抑制浓度 (MIC) 范围为 0.88–10 μM,并且均具有杀真菌活性,最小杀真菌浓度 (MFC) 类似于相应的 MIC 值。化合物24,36,和48是特别有效对抗隐球菌ATCC 90113具有1.0 / 1.0的MIC / MFC值,1.6 / 1.6和1.3 / 2.0μM但显示出低细胞毒性,其IC 50> 40 μM 对抗哺乳动物 Vero 细胞。结构和抗真菌活性的关系表明,韧皮素的合成修饰可以提供具有有效抗真菌活性和降低细胞毒性的类似物,需要对这类新的抗真菌化合物进行进一步的临床前研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号