Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CRISPR-Cas12a-Powered Dual-Mode Biosensor for Ultrasensitive and Cross-validating Detection of Pathogenic Bacteria
ACS Sensors ( IF 8.2 ) Pub Date : 2021-07-19 , DOI: 10.1021/acssensors.1c00686 Long Ma 1 , Lei Peng 1 , Lijuan Yin 1 , Guozhen Liu 2, 3 , Shuli Man 1
ACS Sensors ( IF 8.2 ) Pub Date : 2021-07-19 , DOI: 10.1021/acssensors.1c00686 Long Ma 1 , Lei Peng 1 , Lijuan Yin 1 , Guozhen Liu 2, 3 , Shuli Man 1
Affiliation
|
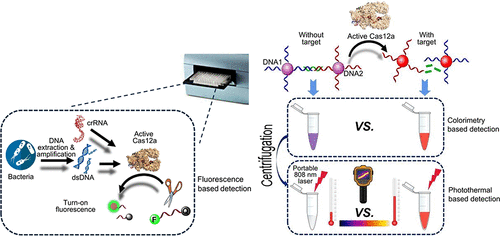
Pathogenic bacteria infection severely threatens human health and causes substantial medical and financial concern. Rapid, sensitive, specific, and reliable detection of pathogenic bacteria is crucial. In the current study, a CRISPR-Cas12a-powered dual-mode biosensor was developed for ultrasensitive and cross-validating detection of pathogenic bacteria. Simply, the amplicons of Salmonella (used as a model)-specific invA sequence triggered CRISPR-Cas12a-based indiscriminate degradation of single-stranded DNAs that were supposed to link two gold nanoparticle (AuNP) probe pairs, inducing an aggregation-to-dispersion change. This generated observable color changes that became even more apparent after centrifugation. The color changes can be discerned by naked eyes and recorded using a portable colorimeter. Meanwhile, the photothermal effect of CRISPR-Cas12-powered AuNPs was explored for the first time through 808 nm near-infrared irradiation such that quantitative measurement can be carried out by recording temperatures using a thermal camera. For both modes, a limit of detection of 1 CFU/mL and a dynamic range of detection from 100 to 108 CFU/mL were obtained, which were comparable with or better than previously reported methods. Our proposed biosensor demonstrated satisfactory selectivity for Salmonella against other interfering cells. Furthermore, this biosensor proved to be capable of Salmonella detection in food samples. Regarding the real applications, the result from each mode can be used for cross-validation. Only the case having doubly confirmed positive or negative results can be assured, which rendered a more dependable biosensing conclusion. This technology not only expands the reach of the CRISPR-Cas system in biosensing but also provides a general method for bacteria sensing with desirable sensitivity, specificity, and cross-validating capacity.
中文翻译:

CRISPR-Cas12a 驱动的双模式生物传感器,用于病原菌的超灵敏和交叉验证检测
病原菌感染严重威胁人类健康并引起严重的医疗和经济问题。快速、灵敏、特异和可靠地检测病原菌至关重要。在目前的研究中,开发了一种 CRISPR-Cas12a 驱动的双模式生物传感器,用于病原菌的超灵敏和交叉验证检测。简单地说,沙门氏菌的扩增子(用作模型)特异性invA序列触发了基于 CRISPR-Cas12a 的单链 DNA 的无差别降解,这些单链 DNA 应该连接两个金纳米颗粒 (AuNP) 探针对,诱导聚集到分散的变化。这产生了可观察到的颜色变化,在离心后变得更加明显。肉眼可以辨别颜色变化,并使用便携式色度计记录。同时,首次通过 808 nm 近红外辐射探索了 CRISPR-Cas12 驱动的 AuNPs 的光热效应,从而可以通过使用热像仪记录温度来进行定量测量。对于这两种模式,检测限为 1 CFU/mL,检测动态范围为 10 0至 10 8获得了 CFU/mL,这与之前报道的方法相当或更好。我们提出的生物传感器对沙门氏菌对其他干扰细胞表现出令人满意的选择性。此外,该生物传感器被证明能够检测食品样品中的沙门氏菌。对于实际应用,每种模式的结果都可以用于交叉验证。只有在双重确认阳性或阴性结果的情况下才能确定,这使得生物传感结论更加可靠。该技术不仅扩展了 CRISPR-Cas 系统在生物传感中的应用范围,而且还提供了一种具有理想灵敏度、特异性和交叉验证能力的细菌传感通用方法。
更新日期:2021-08-27
中文翻译:

CRISPR-Cas12a 驱动的双模式生物传感器,用于病原菌的超灵敏和交叉验证检测
病原菌感染严重威胁人类健康并引起严重的医疗和经济问题。快速、灵敏、特异和可靠地检测病原菌至关重要。在目前的研究中,开发了一种 CRISPR-Cas12a 驱动的双模式生物传感器,用于病原菌的超灵敏和交叉验证检测。简单地说,沙门氏菌的扩增子(用作模型)特异性invA序列触发了基于 CRISPR-Cas12a 的单链 DNA 的无差别降解,这些单链 DNA 应该连接两个金纳米颗粒 (AuNP) 探针对,诱导聚集到分散的变化。这产生了可观察到的颜色变化,在离心后变得更加明显。肉眼可以辨别颜色变化,并使用便携式色度计记录。同时,首次通过 808 nm 近红外辐射探索了 CRISPR-Cas12 驱动的 AuNPs 的光热效应,从而可以通过使用热像仪记录温度来进行定量测量。对于这两种模式,检测限为 1 CFU/mL,检测动态范围为 10 0至 10 8获得了 CFU/mL,这与之前报道的方法相当或更好。我们提出的生物传感器对沙门氏菌对其他干扰细胞表现出令人满意的选择性。此外,该生物传感器被证明能够检测食品样品中的沙门氏菌。对于实际应用,每种模式的结果都可以用于交叉验证。只有在双重确认阳性或阴性结果的情况下才能确定,这使得生物传感结论更加可靠。该技术不仅扩展了 CRISPR-Cas 系统在生物传感中的应用范围,而且还提供了一种具有理想灵敏度、特异性和交叉验证能力的细菌传感通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号