Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-07-20 , DOI: 10.1016/j.atmosenv.2021.118622 Xiang He 1 , Jian-Jun Wu 1 , Zhi-Cheng Ma 1 , Xi Xi 1 , Yun-Hong Zhang 2
|
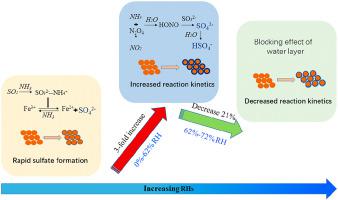
Heterogeneous reactions play an essential role in the research area of formation processes of secondary aerosols. In this study, heterogeneous reactions of NH3/SO2/NO2 with α-Fe2O3 particles at different relative humidities (RHs) were investigated by using a gas-flow system combined with diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). The results show that the presence of NH3 leads to the rapid conversion of SO2 to sulfate as a result of the proton transfer function and neutralization on the α-Fe2O3 particles in dry condition. NH3 can also promote the hydrolysis of NO2 dimers to HONO formation and result in the formation of more sulfate on the particle surface in humid conditions. On the other hand, water vapor plays both positive and negative roles in the uptake of SO2 during the reaction processes. When the RH increases from 0% to 62%, the formation rate of sulfate and uptake coefficient for SO2 in the initial stage increase about 3-fold. However, due to the inhibition effect of water layer between trace gases and active surface, the above values of reaction kinetic decrease about 21% when the RH increases from 62% to 72%. Our results may provide insight into the effects of NH3 and RH for the converting SO2 into sulfate on α-Fe2O3 particles, which supply basic data for the formation of secondary particles in field studies of atmospheric environment.
中文翻译:

NH 3促进了不同相对湿度下NO 2共存的α-Fe 2 O 3颗粒上SO 2生成硫酸盐的多相反应
非均相反应在二次气溶胶形成过程的研究领域发挥着重要作用。在这项研究中,NH 3 /SO 2 /NO 2与α-Fe 2 O 3颗粒在不同相对湿度(RHs)下的多相反应通过使用气流系统结合漫反射红外傅立叶变换光谱(DRIFTS)进行了研究. 结果表明,NH 3的存在导致SO 2快速转化为硫酸盐,这是干燥条件下α-Fe 2 O 3颗粒上的质子传递函数和中和作用的结果。NH 3还可以促进 NO 2二聚体水解成 HONO,并导致在潮湿条件下在颗粒表面形成更多的硫酸盐。另一方面,水蒸气在反应过程中对 SO 2的吸收起着积极和消极的作用。当相对湿度从0%增加到62%时,初期硫酸盐生成率和SO 2吸收系数增加约3倍。然而,由于微量气体与活性表面之间水层的抑制作用,当相对湿度从62%增加到72%时,上述反应动力学值下降约21%。我们的结果可以深入了解 NH 3和 RH 对 SO 2转化为硫酸盐对 α-Fe 的影响2 O 3粒子,为大气环境实地研究中二次粒子的形成提供基础数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号