当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo- and Regioselective Synthesis of Functionalized 1H-imidazo[1,5-a]indol-3(2H)-ones via a Redox-Neutral Rhodium(III)-Catalyzed [4+1] Annulation between Indoles and Alkynes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-19 , DOI: 10.1002/adsc.202100555 Fei Zhao 1 , Jing Chen 2 , Jin Qiao 1 , Yangbin Lu 1 , Xiaoning Zhang 1 , Hui Mao 3 , Shiyao Lu 1 , Xin Gong 1 , Siyu Liu 1 , Xiaowei Wu 4 , Long Dai 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-07-19 , DOI: 10.1002/adsc.202100555 Fei Zhao 1 , Jing Chen 2 , Jin Qiao 1 , Yangbin Lu 1 , Xiaoning Zhang 1 , Hui Mao 3 , Shiyao Lu 1 , Xin Gong 1 , Siyu Liu 1 , Xiaowei Wu 4 , Long Dai 1
Affiliation
|
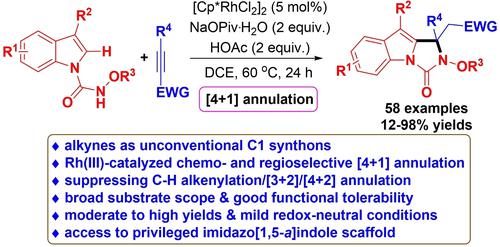
Alkynes generally serve as C2 synthons in transition-metal-catalyzed C−H annulations, herein, exploiting electron-deficient alkynes as unconventional C1 synthons, the chemo- and regiospecific synthesis of functionalized 1H-imidazo[1,5-a]indol-3(2H)-ones via a redox-neutral rhodium(III)-catalyzed [4+1] annulation of N-carbamoyl indoles has been achieved. This process is characterized by high chemo- and regioselectivity, broad substrate scope, good tolerance of functional groups, moderate to high yields and mild redox-neutral conditions, thus affording a robust approach to access valuable 1H-imidazo[1,5-a]indol-3(2H)-ones.
中文翻译:

通过氧化还原中性铑 (III)-催化的吲哚和炔烃之间的 [4+1] 环化反应化学和区域选择性合成功能化 1H-咪唑并 [1,5-a] 吲哚-3(2H)-酮
炔烃通常在过渡金属催化的 C−H 环化中用作 C 2合成子,在此,利用缺电子炔烃作为非常规 C 1合成子,功能化 1 H-咪唑并[1,5- a ]的化学和区域特异性合成indol-3(2 H )-ones 通过氧化还原中性铑 (III) 催化的 [4+1] 环化N-氨基甲酰基吲哚已经实现。该工艺的特点是化学选择性和区域选择性高、底物范围广、官能团耐受性好、产率中等至高和氧化还原中性条件温和,从而为获取有价值的 1 H-咪唑并[1,5- a ]indol-3(2 H)-那些。
更新日期:2021-09-21
中文翻译:

通过氧化还原中性铑 (III)-催化的吲哚和炔烃之间的 [4+1] 环化反应化学和区域选择性合成功能化 1H-咪唑并 [1,5-a] 吲哚-3(2H)-酮
炔烃通常在过渡金属催化的 C−H 环化中用作 C 2合成子,在此,利用缺电子炔烃作为非常规 C 1合成子,功能化 1 H-咪唑并[1,5- a ]的化学和区域特异性合成indol-3(2 H )-ones 通过氧化还原中性铑 (III) 催化的 [4+1] 环化N-氨基甲酰基吲哚已经实现。该工艺的特点是化学选择性和区域选择性高、底物范围广、官能团耐受性好、产率中等至高和氧化还原中性条件温和,从而为获取有价值的 1 H-咪唑并[1,5- a ]indol-3(2 H)-那些。











































 京公网安备 11010802027423号
京公网安备 11010802027423号