当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of rhamnolipid biosurfactant on the dissolution and transport of silver nanoparticles in porous media
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-07-15 , DOI: 10.1039/d1en00185j Shuchi Liao 1 , Chen Liu 1 , Dorothea Pinchbeck 1 , Natalie L. Cápiro 2 , John D. Fortner 3 , Linda M. Abriola 1 , Kurt D. Pennell 1
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-07-15 , DOI: 10.1039/d1en00185j Shuchi Liao 1 , Chen Liu 1 , Dorothea Pinchbeck 1 , Natalie L. Cápiro 2 , John D. Fortner 3 , Linda M. Abriola 1 , Kurt D. Pennell 1
Affiliation
|
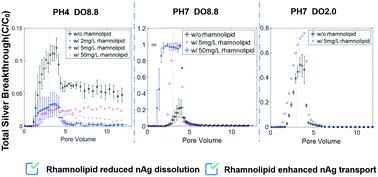
The effects of nanoscale silver (nAg) particles on subsurface microbial communities can be influenced by the presence of biosurfactants, which have been shown to alter nanoparticle surface properties. Batch and column studies were conducted to investigate the influence of rhamnolipid biosurfactant (1–50 mg L−1) on the stability and mobility of silver nanoparticles (16 ± 4 nm) in batch reactors and water-saturated columns with three solution chemistries: pH = 4 and dissolved oxygen concentration (DO) = 8.8 mg L−1, pH = 7 and DO = 8.8 mg L−1, pH = 7 and DO = 2.0 mg L−1. In batch studies, the presence of rhamnolipid (2–50 mg L−1) reduced nAg dissolution by 83.3–99.1% under all pH and DO conditions. Improved nAg stability was observed when rhamnolipid was present in batch reactors at pH = 7 ± 0.2, where the hydrodynamic diameter remained constant (∼50 nm) relative to rhamnolipid-free controls (increased to >230 nm) in 48 hours. Column experiments conducted at pH 4.0 ± 0.2 demonstrated that co-injection of nAg with rhamnolipid (2, 5 and 50 mg L−1) decreased Ag+ breakthrough from ∼22% of total applied mass in rhamnolipid-free columns to less than 8.1% in the presence of rhamnolipid and altered the shape of the nAg retention profile from a hyper-exponential to a uniform distribution. Column experiments performed at pH 7.0 ± 0.2 and DO levels of either ∼2.0 or ∼8.8 mg L−1 showed that co-injection of 5 mg L−1 and 50 mg L−1 rhamnolipid increased nAg mass breakthrough by 25–40% and ∼80%, respectively, enhancements in nAg stability and mobility were attributed to rhamnolipid adsorption on nAg surfaces, which effectively slowed the oxidation and thus release of Ag+, and adsorption of rhamnolipid on the porous medium, which competed for nAg attachment sites. These results indicate that the presence of rhamnolipid significantly influenced nAg dissolution and mobility under dynamic flow conditions. A mathematical model based on modified filtration theory (MFT) accurately reproduced nAg transport and retention behavior when aggregation and reaction processes were minimal and when rhamnolipid was present, providing a tool to predict the effects of biosurfactants on nAg transport in porous media.
中文翻译:

鼠李糖脂生物表面活性剂对银纳米粒子在多孔介质中溶解和迁移的影响
纳米银 (nAg) 颗粒对地下微生物群落的影响可能会受到生物表面活性剂的影响,生物表面活性剂已被证明会改变纳米颗粒的表面特性。进行批次和柱研究以研究鼠李糖脂生物表面活性剂 (1–50 mg L -1 ) 对间歇反应器和水饱和柱中银纳米粒子 (16 ± 4 nm) 稳定性和流动性的影响,具有三种溶液化学物质:pH = 4 且溶解氧浓度 (DO) = 8.8 mg L -1,pH = 7 且溶解氧 = 8.8 mg L -1,pH = 7 且溶解氧 = 2.0 mg L -1。在批量研究中,鼠李糖脂(2–50 mg L -1) 在所有 pH 和 DO 条件下将 nAg 溶解降低了 83.3-99.1%。当鼠李糖脂存在于 pH = 7 ± 0.2 的间歇式反应器中时,观察到 nAg 稳定性提高,其中流体动力学直径在 48 小时内相对于不含鼠李糖脂的对照(增加至 >230 nm)保持恒定(~50 nm)。在 pH 4.0 ± 0.2 下进行的柱实验表明,nAg 与鼠李糖脂(2、5 和 50 mg L -1)的共同注射将Ag +穿透从无鼠李糖脂色谱柱中总应用质量的约 22%降低到低于 8.1%在鼠李糖脂存在的情况下,将 nAg 保留曲线的形状从超指数分布更改为均匀分布。在 pH 7.0 ± 0.2 和 DO 水平为 ~2.0 或 ~8.8 mg L -1 下进行的柱实验表明共注射 5 mg L -1和 50 mg L -1鼠李糖脂分别使 nAg 质量突破增加了 25-40% 和~80%,nAg 稳定性和流动性的增强归因于 nAg 表面上的鼠李糖脂吸附,有效减缓氧化,从而释放 Ag +和鼠李糖脂在多孔介质上的吸附,从而竞争 nAg 附着位点。这些结果表明鼠李糖脂的存在显着影响了动态流动条件下 nAg 的溶解和流动性。当聚集和反应过程最小以及存在鼠李糖脂时,基于改进过滤理论 (MFT) 的数学模型准确再现了 nAg 传输和保留行为,提供了一种工具来预测生物表面活性剂对多孔介质中 nAg 传输的影响。
更新日期:2021-07-20
中文翻译:

鼠李糖脂生物表面活性剂对银纳米粒子在多孔介质中溶解和迁移的影响
纳米银 (nAg) 颗粒对地下微生物群落的影响可能会受到生物表面活性剂的影响,生物表面活性剂已被证明会改变纳米颗粒的表面特性。进行批次和柱研究以研究鼠李糖脂生物表面活性剂 (1–50 mg L -1 ) 对间歇反应器和水饱和柱中银纳米粒子 (16 ± 4 nm) 稳定性和流动性的影响,具有三种溶液化学物质:pH = 4 且溶解氧浓度 (DO) = 8.8 mg L -1,pH = 7 且溶解氧 = 8.8 mg L -1,pH = 7 且溶解氧 = 2.0 mg L -1。在批量研究中,鼠李糖脂(2–50 mg L -1) 在所有 pH 和 DO 条件下将 nAg 溶解降低了 83.3-99.1%。当鼠李糖脂存在于 pH = 7 ± 0.2 的间歇式反应器中时,观察到 nAg 稳定性提高,其中流体动力学直径在 48 小时内相对于不含鼠李糖脂的对照(增加至 >230 nm)保持恒定(~50 nm)。在 pH 4.0 ± 0.2 下进行的柱实验表明,nAg 与鼠李糖脂(2、5 和 50 mg L -1)的共同注射将Ag +穿透从无鼠李糖脂色谱柱中总应用质量的约 22%降低到低于 8.1%在鼠李糖脂存在的情况下,将 nAg 保留曲线的形状从超指数分布更改为均匀分布。在 pH 7.0 ± 0.2 和 DO 水平为 ~2.0 或 ~8.8 mg L -1 下进行的柱实验表明共注射 5 mg L -1和 50 mg L -1鼠李糖脂分别使 nAg 质量突破增加了 25-40% 和~80%,nAg 稳定性和流动性的增强归因于 nAg 表面上的鼠李糖脂吸附,有效减缓氧化,从而释放 Ag +和鼠李糖脂在多孔介质上的吸附,从而竞争 nAg 附着位点。这些结果表明鼠李糖脂的存在显着影响了动态流动条件下 nAg 的溶解和流动性。当聚集和反应过程最小以及存在鼠李糖脂时,基于改进过滤理论 (MFT) 的数学模型准确再现了 nAg 传输和保留行为,提供了一种工具来预测生物表面活性剂对多孔介质中 nAg 传输的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号