当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective Photoreaction of Enamides: Divergent Reactivity towards [3+2]-Photocycloaddition vs Paternò–Büchi Reaction†
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2021-07-19 , DOI: 10.1111/php.13489 Sunil Kumar Kandappa 1, 2 , Elango Kumarasamy 1, 3 , Ravichandranath Singathi 1 , Lakshmy Kannadi Valloli 1 , Angel Ugrinov 4 , Jayaraman Sivaguru 1
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2021-07-19 , DOI: 10.1111/php.13489 Sunil Kumar Kandappa 1, 2 , Elango Kumarasamy 1, 3 , Ravichandranath Singathi 1 , Lakshmy Kannadi Valloli 1 , Angel Ugrinov 4 , Jayaraman Sivaguru 1
Affiliation
|
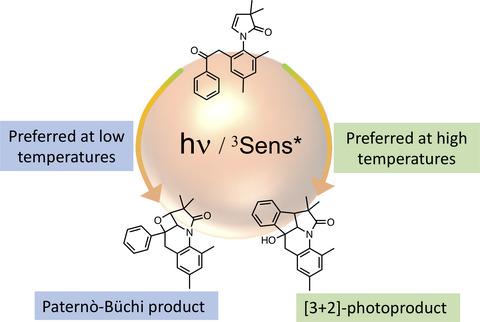
Photoreaction of enamides tethered to a phenyl ketone leads to either [3+2]-photocycloaddition or Paternò–Büchi reaction. This divergence in chemical reactivity originating from the same excited state was dependent on the reaction temperature. At low temperatures the Paternò–Büchi reaction was preferred, whereas at higher temperatures there was preference toward formation of [3+2]-photoproduct.
中文翻译:

烯酰胺的化学选择性光反应:对 [3+2]-光环加成反应与 Paternò–Büchi 反应的不同反应†
与苯基酮结合的烯酰胺的光反应导致[3+2]-光环加成或Paternò-Büchi 反应。这种源自相同激发态的化学反应性差异取决于反应温度。在低温下,Paternò-Büchi 反应是优选的,而在较高温度下,更倾向于形成 [3+2]-光产物。
更新日期:2021-07-19
中文翻译:

烯酰胺的化学选择性光反应:对 [3+2]-光环加成反应与 Paternò–Büchi 反应的不同反应†
与苯基酮结合的烯酰胺的光反应导致[3+2]-光环加成或Paternò-Büchi 反应。这种源自相同激发态的化学反应性差异取决于反应温度。在低温下,Paternò-Büchi 反应是优选的,而在较高温度下,更倾向于形成 [3+2]-光产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号