当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Factors governing the affinity and selectivity of histone deacetylase inhibitors for the HDAC8 enzyme active site: Implications for anticancer therapy
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-07-19 , DOI: 10.1002/poc.4268 Nikolay Toshev 1, 2 , Diana Cheshmedzhieva 1 , Todor Dudev 1
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-07-19 , DOI: 10.1002/poc.4268 Nikolay Toshev 1, 2 , Diana Cheshmedzhieva 1 , Todor Dudev 1
Affiliation
|
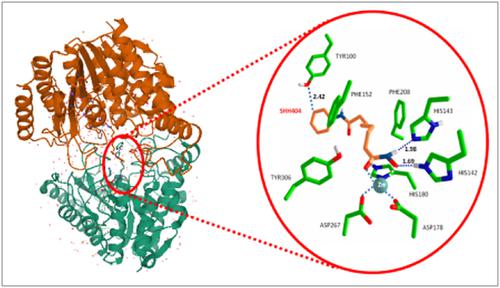
Disruptions in post-translational modifications of chromatin structure promote uncontrollable cell growth branded as a hallmark of tumor lesions. The overexpression/hyperactivity of histone deacetylases (HDACs) is a common feature for the tumorogenesis and cancer progression. Several inhibitors of histone deacetylases (mainly hydroxamic acid derivatives) have been successfully used as drugs in fighting tumor formations. However, there is no systematic study on the factors controlling the affinity and selectivity of this type of inhibitors to the host enzyme thus hampering successful rational design of more potent and selective anticancer drugs. Herein, in an attempt to unravel the mechanism of the host–guest interactions in these systems at atomic level, we systematically study the effect of various factors in the process and elucidate its key determinants. Density functional theory calculations have been employed. Our findings have the potential to be employed as guidelines in designing new HDAC inhibitors with improved anticancer properties.
中文翻译:

控制组蛋白去乙酰化酶抑制剂对 HDAC8 酶活性位点的亲和力和选择性的因素:对抗癌治疗的影响
染色质结构翻译后修饰的破坏促进了不可控制的细胞生长,被称为肿瘤病变的标志。组蛋白去乙酰化酶 (HDAC) 的过度表达/过度活跃是肿瘤发生和癌症进展的共同特征。几种组蛋白去乙酰化酶抑制剂(主要是异羟肟酸衍生物)已成功用作对抗肿瘤形成的药物。然而,没有系统研究控制此类抑制剂对宿主酶的亲和力和选择性的因素,从而阻碍了更有效和选择性的抗癌药物的成功合理设计。在这里,为了在原子水平上解开这些系统中主客交互的机制,我们系统地研究了该过程中各种因素的影响并阐明了其关键决定因素。已采用密度泛函理论计算。我们的发现有可能被用作设计具有改进抗癌特性的新 HDAC 抑制剂的指南。
更新日期:2021-07-19
中文翻译:

控制组蛋白去乙酰化酶抑制剂对 HDAC8 酶活性位点的亲和力和选择性的因素:对抗癌治疗的影响
染色质结构翻译后修饰的破坏促进了不可控制的细胞生长,被称为肿瘤病变的标志。组蛋白去乙酰化酶 (HDAC) 的过度表达/过度活跃是肿瘤发生和癌症进展的共同特征。几种组蛋白去乙酰化酶抑制剂(主要是异羟肟酸衍生物)已成功用作对抗肿瘤形成的药物。然而,没有系统研究控制此类抑制剂对宿主酶的亲和力和选择性的因素,从而阻碍了更有效和选择性的抗癌药物的成功合理设计。在这里,为了在原子水平上解开这些系统中主客交互的机制,我们系统地研究了该过程中各种因素的影响并阐明了其关键决定因素。已采用密度泛函理论计算。我们的发现有可能被用作设计具有改进抗癌特性的新 HDAC 抑制剂的指南。



























 京公网安备 11010802027423号
京公网安备 11010802027423号