Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of (1R,5S)-2-methyl-6,7-benzomorphan via Aza-Prins reaction
Chirality ( IF 2.8 ) Pub Date : 2021-07-19 , DOI: 10.1002/chir.23338 José A Gálvez 1 , Ramón Badorrey 1 , Alejandro Mahía 2, 3 , María D Díaz-de-Villegas 1
Chirality ( IF 2.8 ) Pub Date : 2021-07-19 , DOI: 10.1002/chir.23338 José A Gálvez 1 , Ramón Badorrey 1 , Alejandro Mahía 2, 3 , María D Díaz-de-Villegas 1
Affiliation
|
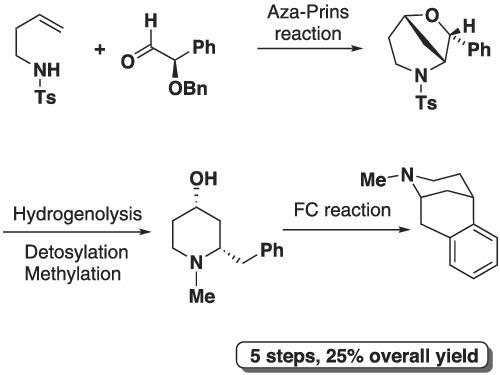
(1R,5S)-2-Methyl-6,7-benzomorphan has been synthesised from (R)-(benzyloxy)(phenyl)acetaldehyde. On a 2-mmol scale Bi (OTf)3 promoted Aza-Prins reaction with N-tosylhomoallylamine afforded an 88/12 mixture of 6-oxa-2-azabicyclo[3.2.1]octanes. Major diastereoisomer was converted to enantiomerically pure (2S,4S)-2-benzyl-1- methylpiperidin-4-ol via a high-yielding sequence hydrogenolysis/N-detosylation/N-methylation. Acid-catalysed intramolecular Friedel−Crafts cyclisation of the piperidinol afforded (1R,5S)-2-methyl-6,7-benzomorphan in five steps with a yield of 25%.
中文翻译:

通过 Aza-Prins 反应不对称合成 (1R,5S)-2-methyl-6,7-benzomorphan
(1 R ,5 S )-2-Methyl-6,7-benzomorphan 由 ( R )-(苄氧基)(苯基)乙醛合成。在 2 mmol 规模上,Bi (OTf) 3促进了 Aza-Prins 与N-甲苯磺酰基高烯丙胺的反应,得到 6-oxa-2-azabicyclo[3.2.1] 辛烷的 88/12 混合物。主要的非对映异构体通过高产率的氢解/N-去甲苯基化/N-甲基化序列转化为对映体纯的 (2 S ,4 S )-2-苄基-1-甲基哌啶-4-醇。哌啶醇的酸催化分子内 Friedel-Crafts 环化分五步得到 (1 R ,5 S )-2-甲基-6,7-苯并吗喃,产率为 25%。
更新日期:2021-08-12
中文翻译:

通过 Aza-Prins 反应不对称合成 (1R,5S)-2-methyl-6,7-benzomorphan
(1 R ,5 S )-2-Methyl-6,7-benzomorphan 由 ( R )-(苄氧基)(苯基)乙醛合成。在 2 mmol 规模上,Bi (OTf) 3促进了 Aza-Prins 与N-甲苯磺酰基高烯丙胺的反应,得到 6-oxa-2-azabicyclo[3.2.1] 辛烷的 88/12 混合物。主要的非对映异构体通过高产率的氢解/N-去甲苯基化/N-甲基化序列转化为对映体纯的 (2 S ,4 S )-2-苄基-1-甲基哌啶-4-醇。哌啶醇的酸催化分子内 Friedel-Crafts 环化分五步得到 (1 R ,5 S )-2-甲基-6,7-苯并吗喃,产率为 25%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号