当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General Strategy for the Stereoselective Synthesis of Pyrrole-Fused Chiral Skeletons: [3+2] Cycloaddition with 2-Nitro-2,3-Unsaturated Glycosides
ChemCatChem ( IF 3.8 ) Pub Date : 2021-07-18 , DOI: 10.1002/cctc.202100795 Nan Jiang 1 , Yuling Mei 1 , Yu Yang 1 , Youxian Dong 1 , Zekun Ding 1 , Jianbo Zhang 2
ChemCatChem ( IF 3.8 ) Pub Date : 2021-07-18 , DOI: 10.1002/cctc.202100795 Nan Jiang 1 , Yuling Mei 1 , Yu Yang 1 , Youxian Dong 1 , Zekun Ding 1 , Jianbo Zhang 2
Affiliation
|
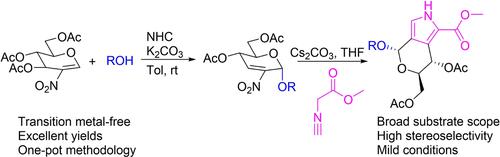
In this study, a two-step methodology was developed for the synthesis of chiral pyrroles from 2-nitroglycals via Ferrier rearrangement and Barton-Zard reaction under mild conditions without transition metal catalysts. The Ferrier rearrangement reaction of 2-nitro-glycals and a series of O-nucleophiles proceeded smoothly in the presence of N-heterocyclic carbene (NHC) catalyst and K2CO3 which allowed the highly stereoselective synthesis of the diverse 2-nitro-2,3-unsaturated glycosides in excellent yields. Subsequently, the rearrangement products were conveniently transformed into the desired chiral pyrroles by [3+2] cycloaddition (Barton-Zard reaction) with isocyanoacetate in the presence of Cs2CO3. One-pot strategy was also successfully demonstrated for the gram-scale synthesis of one chiral pyrrole. This is the first report of stereoselective conversion from 2-nitroglycals to chiral pyrrole.
中文翻译:

吡咯稠合手性骨架立体选择性合成的一般策略:[3+2] 环加成与 2-硝基-2,3-不饱和糖苷
在这项研究中,开发了一种两步法,用于在没有过渡金属催化剂的温和条件下,通过Ferrier 重排和 Barton-Zard 反应从 2-硝基糖类合成手性吡咯。在N-杂环卡宾 (NHC) 催化剂和 K 2 CO 3的存在下,2-硝基-乙二醇和一系列O-亲核试剂的费里尔重排反应顺利进行,这使得各种 2-硝基-2 的高度立体选择性合成成为可能。 ,3-不饱和糖苷,产率极好。随后,在 Cs 2存在下,通过与异氰乙酸酯的 [3+2] 环加成(Barton-Zard 反应),将重排产物方便地转化为所需的手性吡咯一氧化碳3。一种手性吡咯的克级合成也成功地证明了一锅法。这是从 2-硝基乙二醇到手性吡咯的立体选择性转化的第一份报告。
更新日期:2021-09-17
中文翻译:

吡咯稠合手性骨架立体选择性合成的一般策略:[3+2] 环加成与 2-硝基-2,3-不饱和糖苷
在这项研究中,开发了一种两步法,用于在没有过渡金属催化剂的温和条件下,通过Ferrier 重排和 Barton-Zard 反应从 2-硝基糖类合成手性吡咯。在N-杂环卡宾 (NHC) 催化剂和 K 2 CO 3的存在下,2-硝基-乙二醇和一系列O-亲核试剂的费里尔重排反应顺利进行,这使得各种 2-硝基-2 的高度立体选择性合成成为可能。 ,3-不饱和糖苷,产率极好。随后,在 Cs 2存在下,通过与异氰乙酸酯的 [3+2] 环加成(Barton-Zard 反应),将重排产物方便地转化为所需的手性吡咯一氧化碳3。一种手性吡咯的克级合成也成功地证明了一锅法。这是从 2-硝基乙二醇到手性吡咯的立体选择性转化的第一份报告。









































 京公网安备 11010802027423号
京公网安备 11010802027423号